

D. J. Campbell, K. J. Beckman, C. E. Calderon, P. W. Doolan, R. H. Moore,
A. B. Ellis, G. C. Lisensky,
"Replication and Compression of Bulk Surface Structures with Polydimethylsiloxane
Elastomer."
J. Chem. Educ. Vol. 76 , 537(1999)
This site details a few of the many experiments and demonstrations that can be performed with the elastomer polydimethylsiloxane (PDMS). We recommend Dow Corning Sylgard Elastomer 184, which comes in two parts: a base and a curing agent. The elastomer is available as a half-kilogram kit from Dow Corning (currently 5 kits can be purchased for about $40 apiece). The kit components are fairly harmless, but should not be ingested or allowed to contact the eye. Contact of the kit components with strong acids, bases, or catalytic oxidizing materials may generate hydrogen gas and should be avoided. The cured elastomer is transparent and colorless and, according to Dow Corning, is "ideally suited for electrical/electronic potting and encapsulating applications."
PDMS is cured by an organometallic crosslinking reaction. The siloxane base oligomers contain vinyl groups. The cross-linking oligomers contain at least 3 silicon hydride bonds each. The curing agent contains a proprietary platinum-based catalyst that catalyzes the addition of the SiH bond across the vinyl groups, forming Si-CH2-CH2-Si linkages. The multiple reaction sites on both the base and crosslinking oligomers allow for three-dimensional crosslinking. One advantage of this type of addition reaction is that no waste products such as water are generated. If the ratio of curing agent to base is increased, a harder, more cross-linked elastomer results. Heating will also accelerate the crosslinking reaction.
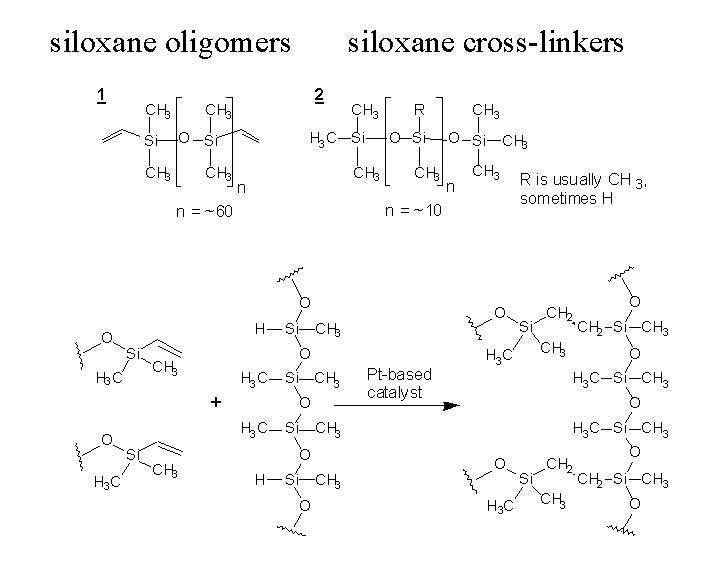
George Whitesides' group at Harvard University has perfected the fabrication of PDMS as a stamp that can be used to pattern areas of metals with thiols. These patterned monolayers can be used to protect areas of the metal surface for lithography or they can be used as patterned templates for cell adhesion. The following experiments give some ideas of the properties of PDMS.
If you have questions or comments about these experiments, contact Dr. Dean Campbell.