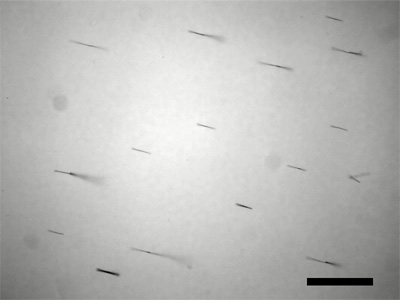
Synthesis of Nickel Nanowires
Procedure modified by J. Whitsett, S. M. Condren, and G. Lisensky from A. K. Bentley, M. Farhoud, A. B. Ellis, G. C. Lisensky, Anne-Marie Nickel, and W. C. Crone, "Template Synthesis and Magnetic Manipulation of Nickel Nanowires," Journal of Chemical Education, 82, 765-768 (2005). Thanks to Anupam Ghosh for suggesting the ethanol storage. This version of the experiment uses a syringe holder, clamps, and an o-ring to hold the filter. It uses more equipment than another version that uses electrical tape but the manipulation is easier.
A simple way to make nanowires is to use a mold or template. In this experiment nickel nanowires are grown inside the pores of an alumina filter and then the filter is removed by etching to yield magnetic nanowires. Nanoporous membranes were designed for health care applications including virus filtration, sample preparation, and liposome manufacture (anodisc filters). These alumina membranes are manufactured by applying a large electrical potential to a piece of aluminum metal submerged in an acid. Aluminum is oxidized to alumina (Al2O3) and pores are created. The size of the pores depends on the potential applied during their creation.
What is the chemical reaction to create the nanowires? Where do the reactants come from? What size and how many nanowires will you make? Are the nanowires crystals? Are they oriented?
| Procedure | Wear eye protection |
Chemical gloves recommended |
 Fumehood recommended Fumehood recommended |
Avoid contact with or inhalation of nickel and nickel solutions. |
Place the disc, metal coated side down, on the copper electrode.
Place the o-ring on the disc.
Stand the cut-off syringe barrel on the o-ring. Firmly hold the barrel in place while securing opposite sides of the barrel with binder clamps. Look down the barrel of the syringe to make sure it is centered over the o-ring.
Record the dry weight of the nickel electrode before electrolysis then insert the nickel electrode into the aligator clip. What is the measured voltage of your 1.5 V battery? Insert the battery into the battery holder, paying attention to polarity. The positive end should be connected to the nickel electrode. Add nickel plating solution to the barrel up to the first markings. Check for leaks. Connect the negative lead of the battery to a multimeter set to read current and then use a jumper cable to connect the multimeter to the copper stage. Insert the plunger into the barrel so the nickel electrode is in the nickel solution, start timing, and record the current passing through the circuit. If no current flows, examine the apparatus to fix any bad electrical connections or replace the current meter. Electrolyze for 20-50 minutes. Record the current every few minutes. What is the average current reading? Longer times give longer wires and better results unless the pore length is exceeded. How many minutes did you use? Disconnect the battery from the copper electrode. Remove the nickel electrode from solution.
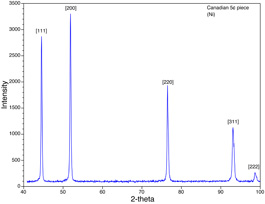
The nickel solution can be reused for this experiment. Why does the concentration of nickel in solution not change during the electrolysis? Rinse the disc with water. Wait for the nickel electrode to dry and measure its dry weight. Firmly hold the barrel in place and remove the binder clips. Transfer the disc from the copper electrode and gently tape the disc shiny side up to a glass slide for removal of the metal coating. In a fume hood, use concentrated nitric acid and a cotton applicator to remove the shiny GaIn or the Ag coating. Soak the cotton applicator in water before disposal. Rinse with water to remove the nitric acid. Even medium concentrations of nitric acid may slowly dissolve nickel. Obtain the powder x-ray diffraction spectrum (2θ = 40-80°, step width 0.05, count 1.0s) of the nickel nanowires in the filter. Place the wet filter on a glass microscope slide as a holder. Which side should be up? How similar is the spectrum to that of nickel metal? Place the disc in about 5 mL of 6 M NaOH (enough to cover the filter) for at least 10 minutes (plastic beaker preferred.) The ceramic material will dissolve. Discard the polymer support ring. Place the beaker on a strong magnet. The nickel nanorods will be attracted towards the magnet. Pour off the NaOH solution. Add water to rinse, place the beaker on a strong magnet, and remove the rinse solution. Repeat many times. (A common error in this experiment is insufficient rinsing.) Use pH paper to check if you have rinsed enough. Transfer the final suspension to a vial for storage. Keep the wires in solution. Obtain the powder x-ray diffraction spectrum (2θ = 40-100°) of the free nickel nanowires. To prepare the sample, wrap a piece of transparent tape very tightly around a microscope slide with the sticky side out. Use an XRD holder to position the tape at the correct distance from the end of the slide.
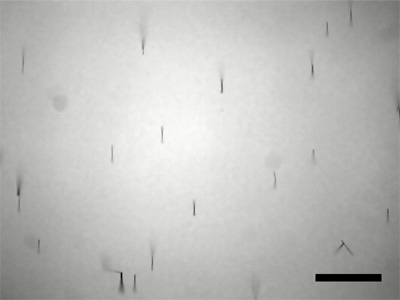
Puddle a fairly dense water suspension of the nanowires onto the tape and let dry enough that the wires stay put when tilted. If you do not have much sample, a strip oriented as shown in the figure is your best chance of getting data. Use an SEM to measure the length of the nanowires. Does the length correlate with deposition time? (Rinsing with ethanol and storing the nanowires in ethanol gives a suspension that leaves less residue and evaporates more quickly for preparation of samples.) To prepare the sample, first examine a nanowire solution under a visible microscope to check concentration. You want to be able to see individual nanowires rather than a mass of nanowires. Dilute the solution if necessary. Transfer the solution from the microscope to a small piece of silicon wafer and let the solution evaporate.
Conclusions
- Based on your reported electrolysis time and the average current, how many grams of nickel did you make? How does this value compare with the change in weight of the electrode?
- How long are your nickel nanowires? You should measure this many times on more than one SEM image, take an average, and report all measured data. (ImageJ is useful for measuring lengths. Use the straight line tool to drag the length of the scale bar and menu Analyze/Set Scale to tell the program the actual length of the scale bar. You can then use the straight line tool to measure lengths in real units.) If the diameter of the wire is 200 nm and the density of nickel is 8.9g/cm3, how many wires did you make?
- Is your product magnetic? How do you know? Report all observations relevant to your answer.
- How could this apparatus be used to make nanowire barcodes? Provide a reference for your answer.
- Does the XRD data show any evidence for a preferred crystal orientation of the nanowires in the filter? Which side of the filter did you examine with the x-rays? What is the ratio of the XRD (220) to (111) peak heights for nickel nanowires in the template, for free nickel nanowires, and for normal nickel metal?
- GE Whatman Anodisc alumina filters 25mm with polypropylene support rings and 0.02 micrometer pores: Fisher Scientific (#09-926-34) or VWR International (#28138-067). Unfortunately the price has gone up to $20/filter.
- GaIn Eutectic: Aldrich (49542-5)
- Ni wire: VWR International (#AA41361-G6) or Alfa Aesar (14337-G6) 1 mm diameter x 10 m long. CAUTION: Avoid physical contact (especially inhalation) with nickel and nickel solutions as nickel is an irritant and carcinogen.
- "Watts" Nickel plating solution: 300g/L NiSO4.6H2O, 45g/L H3BO3 and 45g/L NiCl2.6H2O. (Oliver P. Watts was an electrochemist at the University of Wisconsin who studied nickel plating 100 years ago.)
- Nitric acid (conc), 6 M NaOH
Equipment
- Tweezers, cotton swabs, water wash bottle, beaker
- Strong magnet
- 0.032 inch thick satin finish copper sheet, 12" x 12", McMaster-Carr, (#9801K11)
- 1-1/8" OD x 7/8" ID x 1/8" W o-ring (same diameter as the 1" syringe), Ace Hardware or McMaster-Carr, (#9452K32)
- 1-1/4" binder clips, Staples, (831602)
- 2-1/4" acrylic square, 0.10" thick
- Wooden support blocks made from a 1x2: one piece 3" long, one piece 2-1/2" long and cut in half lengthwise. Screw the acrylic square to the blocks using a 1-1/4" round head screw and washer.
- 30 mL plastic syringes, VWR International, (66064-760). Use a saw to cut the syringe at the 15 mL mark and cut the plunger at the support closest to the end.
- AA Battery holders: Mouser (#534-139)
- Make a hole in the end of the plunger for the positive lead of the battery holder.
- Wires with alligator clips, Amazon.
To prepare the battery holders, cut in half a wire with aligator clips on each end, strip off the insulation for a short distance, use a soldering iron to melt some solder onto each wire and onto the ends of the battery holder, and then use a soldering iron to connect the wires to the battery holder.
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified February 26, 2020.