Synthesis of Nickel Nanowires
Procedure from A. K. Bentley, M. Farhoud, A. B. Ellis, G. C. Lisensky, Anne-Marie Nickel, and W. C. Crone, "Template Synthesis and Magnetic Manipulation of Nickel Nanowires," Journal of Chemical Education, 82, 765-768 (2005). Thanks to Anupam Ghosh for suggesting option C. This version of the experiment uses electrical tape to hold the filter. It uses less equipment but taping the filters without breaking them requires careful manipulation. Another version uses a syringe holder, clamps, and an o-ring instead of taping.
A simple way to make nanowires is to use a mold or template. In this experiment nickel nanowires are grown inside the pores of an alumina filter and then the filter is removed by etching to yield magnetic nanowires.
Nanoporous membranes were designed for health care applications including virus filtration, sample preparation, and liposome manufacture (http://www.whatman.com). These alumina membranes are manufactured by applying a large electrical potential to a piece of aluminum metal submerged in an acid. Aluminum is oxidized to alumina (Al2O3) and pores are created. The size of the pores depends on the applied potential.
| Procedure | Wear eye protection |
Chemical gloves recommended |
 Fumehood recommended |
Avoid contact with or inhalation of nickel and nickel solutions. |
Obtain a 0.02 micrometer Anodisc filter. These ceramic discs are quite brittle and are supported by a polymer ring. Always use tweezers to hold the membranes by the support ring; the alumina will crack if handled directly. Remove the disc from the packaging, remembering which side was up in the box. Fully coat the upper side (the polymer ring looks wider) with a conducting metal (see options in next steps). One option is to use a cotton applicator and liquid GaIn alloy to paint the surface. The coated side will look shiny (and the opposite side will remain lighter.) While it is important to fully coat the surface to prevent leaks, it is only necessary to dip the applicator in the GaIn once. The GaIn can be spread quite thin. Check for gaps in the GaIn coating by looking at the non-coated face of the membrane. Any areas without GaIn will appear light blue in color while areas with GaIn will appear white or opaque. Another option is to sputter Ag metal onto the surface. Conditions used were 50 millitorr argon, 45 milliamps current, for three 150 second depositions. The coated side will look shiny (and the opposite side will remain lighter.)
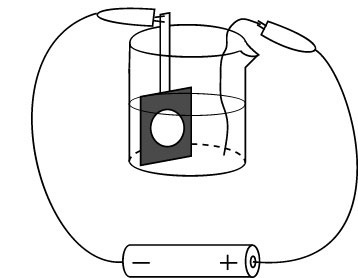
The nickel solution can be reused for this experiment.Why does the concentration of nickel in solution not change during the electrolysis? Rinse the electrode with water. Immerse the electrode in acetone to remove the adhesive. The tape will easily come off the electrode in a few miniutes. (The second movie is time lapse photography and represents 15 minutes actual time.) Remove the disc from the copper electrode and tape shiny side up to a glass slide for removal of the metal coating. In a fume hood, use concentrated nitric acid and a cotton applicator to remove the shiny GaIn or the Ag coating. Soak the cotton applicator in water before disposal. Rinse with water.
Option A: Obtain the powder x-ray diffraction spectrum (2θ = 40-100°) of the nickel nanowires in the filter. Place the disc in 5 mL of 6 M NaOH for 10 minutes. The ceramic material will dissolve. Discard the polymer support ring. Place the beaker on a strong magnet. The nickel nanorods will be attracted towards the magnet. Remove the NaOH solution. Add water to rinse, place the beaker on a strong magnet, and remove the rinse solution. Repeat several times. Transfer the final suspension to a vial for storage. Keep the wires in solution.
Option A: Obtain the powder x-ray diffraction spectrum (2θ = 40-100°) of the free nickel nanowires.
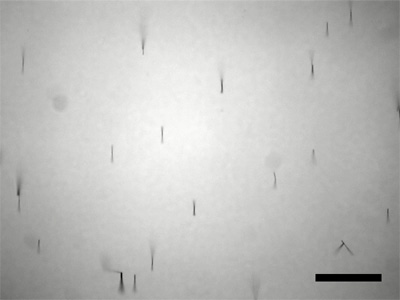
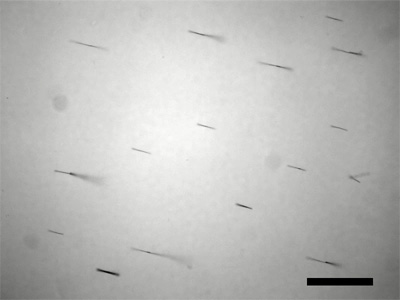
Option B: Use an SEM to measure the length of the nanowires. Does the length correlate with deposition time?
Option C: Rinsing with ethanol (and storing the nanowires in ethanol) gives a suspension that leaves less residue and that evaporates more quickly for preparation of SEM samples.
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified February 6, 2017 .