A Microfluidic Nanofilter
This experiment was developed by Ken Lux and was inspired by the research on "virtual walls" in microfluidic devices by Prof. David Beebe (University of Wisconsin - Madison) and Prof. Jeffrey Moore (University of Illinois - Urbana/Champaign):
Microfluidic devices are a new type of technology that can detect
very small quantities of a substance in a fluid stream. Although the devices
themselves are often large enough to pick up with your hands, the channel height
is just a few times taller than the diameter of a single strand of hair. The
microscale creates special flow characteristics in fluid that passes through
the channel. Substances as small as a few nanometers can be detected in the
fluid. Engineers and scientists have been able to produce microfluidic devices
that detect signs of certain types of cancer in a blood sample before traditional
methods can. Sensors have also been produced that detect environmental contaminants
in water samples. Often these devices incorporate filter mechanisms that remove
unwanted particles from solution or concentrate small particles such as cells
and proteins so that they can be more effectively studied.
This experiment will allow you to create a microfluidic device for the filtration
of an aqueous suspension of PMMA nanoparticles and give you the opportunity
to investigate several nanotechnology and microfluidic phenomena. The device
utilizes a nylon membrane synthesized by a polymerization reaction at the interface
between two immiscible solutions at the "virtual wall" where the hydrophilic
and hydrophobic surface regions in your device intersect.
| Procedure | Wear eye protection |
Chemical gloves recommended |
Preparing the Virtual Wall and Contact Angle Measurement
Prepare an active silver solution.Add concentrated ammonium hydroxide dropwise to 10 mL of 0.1 M silver nitrate solution until the initial precipitate just dissolves. Add 5 mL of 0.8 M KOH solution; a dark precipitate will form. Add more ammonium hydroxide dropwise until the precipitate just redissolves. This "active silver" solution should be used within an hour of preparation. To avoid the formation of explosive silver nitride, discard any remaining active solution by washing down the drain with plenty of water. Deposit silver on a glass slide.
Using tweezers, place a clean microscope slide in a Petri dish. Add 12 drops of 0.5 M glucose solution and 40 drops of active-silver-ion solution onto the slide Gently agitate the Petri dish for several minutes to mix the solutions. A dark precipitate will begin to form and a silver coating will deposit on the glass. Rinse the slide with pure water to reveal the silver coating. Avoid contact with the solution which will stain your hands. Using another slide as a straightedge at a 45 degree angle, scrape off some silver coating with a razor blade.
 |
 |
 |
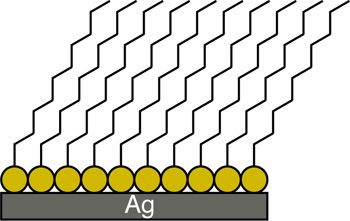
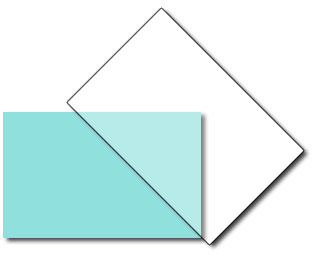
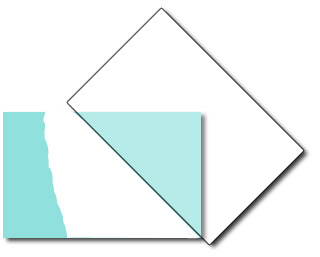
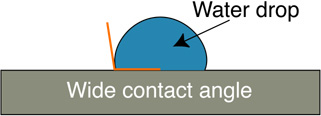
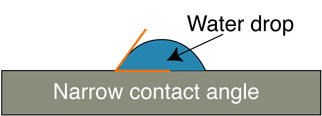
Apply several drops of an ethanol solution of hexadecanethiol to cover the sharp edged silver surface. Allow the ethanol to evaporate, leaving behind an alkanethiol monolayer with the sulfur atoms bound to the silver and the hydrocarbon tails pointing away. Rinse the glass with pure water to remove any hexadecanethiol from the unsilvered region. Because the glass surface is hydrophilic and the functionalized silver surface is hydrophobic, a “virtual wall” is observed at the interface between the functionalized and unfunctionalized surfaces. This interface will be used to position the location of a nylon membrane. A water droplet “beads-up” on the functionalized surface but spreads out on the glass surface. One way to describe this is the contact angle between the drop and the slide. Is the contact angle wide (small attraction to the slide) or narrow (large attraction to the slide)?
 |  |
Constructing the Microchannels and Fluid Ports
Prepare epoxy in a disposable container by mixing equal amounts of the two chemicals from the commercial dispenser. Prepare no more than you can use in the five minutes before it hardens. Thoroughly mix the two chemicals. Create channels for the microfluidic device.Coat one side of a microscope cover slip with epoxy. Form channels by gluing four cover slips to the slide. The interface between the hydrophobic and the hydrophilic regions should run diagonally through the middle of the channel intersection. Glue a fifth cover slip to serve as a "roof" for the channels.
 |
 |
Add connectors for fluid introduction.
Cut a pipet tip on an angle. Epoxy the pipet tip to the device such that the large opening bridges the channel and allows for fluid to flow from the tip into the channel. Seal the edges around the tip to the top cover slip, the lower cover slips, and the original slide.
Synthesizing Polymethacrylate Spheres
Procedure modified by George Lisensky and Jacob Horger, Beloit College, from the Inverse Opal Photonic Crystals Laboratory Guide by R. Schroden and N. Balakrishnan, University of Minnesota MRSEC, 2001.
Monodisperse polymethylmethacrylate (PMMA) spheres are synthesized from a stirred aqueous suspension of methyl methacrylate. The small uniform diameter particles appear irridescent since their size is similar to the wavelength of visible light. Two PMMA suspensions are synthesized, one at a high speed stirring and one at a lower speed.
Note: Once you add the precursor for the polymerization initiator you can move on to the next part while you are waiting for the polymerization to complete.
For both reaction setups, under a slow flow of nitrogen, stir 16 mL pure water with a 20x10 mm oval-shaped magnetic stir bar in a 25 mL round bottom flask equipped with a teflon sleeve and a condenser. The nitrogen enters through a long needle and exits through a short needle passing through a septum in the top of the condenser and then to a bubbler to monitor flow. A Corning stirrer-hotplate spins the stir bar at maximum speed for the 'high speed' reaction and at level 3 for the 'low speed' reaction. A water bath made from a crystalization dish keeps the temperature at 70 degrees C for both. To both reactions, add 3.0 mL methyl methacrylate. Maintain the 70 degree C temperature . The size of the spheres produced depend on temperature, stir rate, and concentration. To both reactions, add 0.015 to 0.020 g 2,2-azobis(2-methylpropionamidine) which decomposes with heat to produce a free radical initiator for the polymerization reaction. (The initiator is added through the condensor since adding it to a joint exposed to methyl methacrylate vapor twice resulted in a permanently polymethylmethacrylate fused joint.) A milky white suspension is observed as the polymerization proceeds. Keep adjusting the temperature to maintain 70 degrees C for the next 40 minutes. After 40 minutes of heating, remove the condenser, and transfer the suspension to vials for storage. Be sure to label which reaction the vials came from. There should not be a noticeable odor if the polymerization was successful.Forming a Nylon-Epoxy Composite Membrane
Synthesize a nylon-epoxy composite membrane at the interface.Connect a short piece of tygon tubing to a plastic syringe. Use the syringe to draw a small amount of aqueous 1,6-diaminohexane/epxoy hardener into the tubing. Connect the tubing to a pipet tip that feeds one of the hydrophilic uncoated glass channels. Very gently apply pressure to the plunger so that solution advances to the interface.(If solution crosses the interface, use the plunger to adjust the solution position.)
Connect another short piece of tygon tubing to a plastic syringe. Use the syringe to draw a small amount of an adipoyl chloride/epoxy resin solution in xylene into the tubing. (Careful: Xylene will react with the syringe.) Connect the tubing to a pipet tip that feeds the opposite hydrophobic coated glass channel. Very gently apply pressure to the plunger so that solution advances to the interface. (A nylon membrane will form when the two solutions meet, hopefully at the interface.)
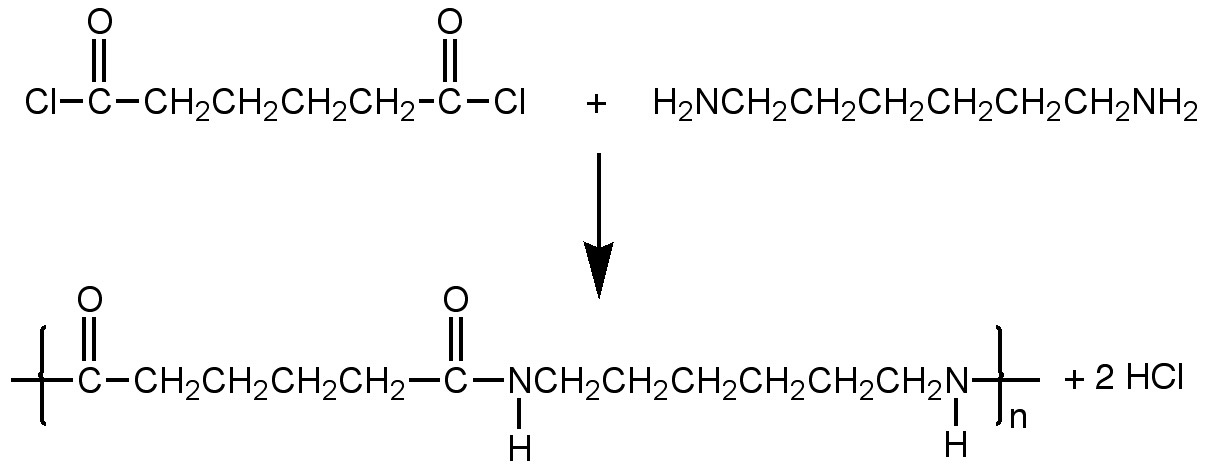
Make two caps for the pipette tips by sealing the end of a short piece of tygon tubing with epoxy.
Filtering
Add 6 drops of the high speed PMMA suspension and 6 drops of the low speed suspension made above to 120 mL of pure water.
Filtration of the PMMA spheres.
Use the two caps you made above to ensure that the only path for solution
flow is across the interface and membrane.
Connect a short piece of tygon tubing to a plastic syringe. Use the syringe
to draw some of the diluted PMMA suspension into the tubing. Connect the
tubing to a pipet tip. Gently apply pressure to the plunger so that solution
advances through the membrane and collect the effluent.
Filtering Efficacy: Diffraction from PMMA Nanopsheres
Follow the procedure for diffraction from PMMA nanospheres to calculate the particle size of the obtained spheres. Evaporate four samples: high speed unfiltered; low speed unfiltered; mixed sample unfiltered; mixed sample filtered.
| 0.5 M glucose | Dissolve 0.90 g glucose in 10 mL of water. |
| 0.8 M KOH | Dissolve 0.45 g KOH in 10 mL of water. |
| 0.1 M silver nitrate | Dissolve 0.17 g AgNO3 in 10 mL of water. |
| 15 M concentrated ammonium hydroxide | |
| Petri dish | |
| alkanethiol solution | Add a few drops of a long-chain alkanethiol (such as octadecanethiol) to 20 mL of ethanol. |
| epoxy | Devcon 5 minute epoxy. |
| Microscope slides and cover slips | We used 3 x 2 inch slides and square cover slips. |
| Micropipet tips | |
| 1,6-diaminohexane epoxy hardener solution | Squeeze a nickel-sized dollop of epoxy hardener into a 100 mL beaker. Add a stir bar and 100 mL water to the beaker and 5 mL of methanol. Stir while heating to suspend epoxy hardener (a cloudy white suspension will result). Use equal parts of this suspension and 60 mM 1,6-diaminohexane in water as the aqueous reactant. |
| acid chloride epoxy resin solution | Squeeze a nickel-sized dollop of epoxy resin into a 100 mL beaker. Add 50 mL of xylenes and stir until the resin is dissolved in the xylenes. Use equal parts of this suspension and 47 mM adipoyl chloride in xylenes as the organic reactant. |
| Plastic tubing and syringe | 5 mL plastic syringes |
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified June 16, 2013 .