Suspension polymerization and size determination of monodispersed polymethylmethacrylate (PMMA) nanospheres
Monodispersed polymethylmethacrylate nanospheres are synthesized from the suspension polymerization of a rapidly stirred aqueous suspension of methyl methacrylate. 2,2-Azobis(2-methylpropionamidine) dihydrochloride is a heat activated water soluble free radical initiator so the emulsion droplets polymerize starting from their outer edge.
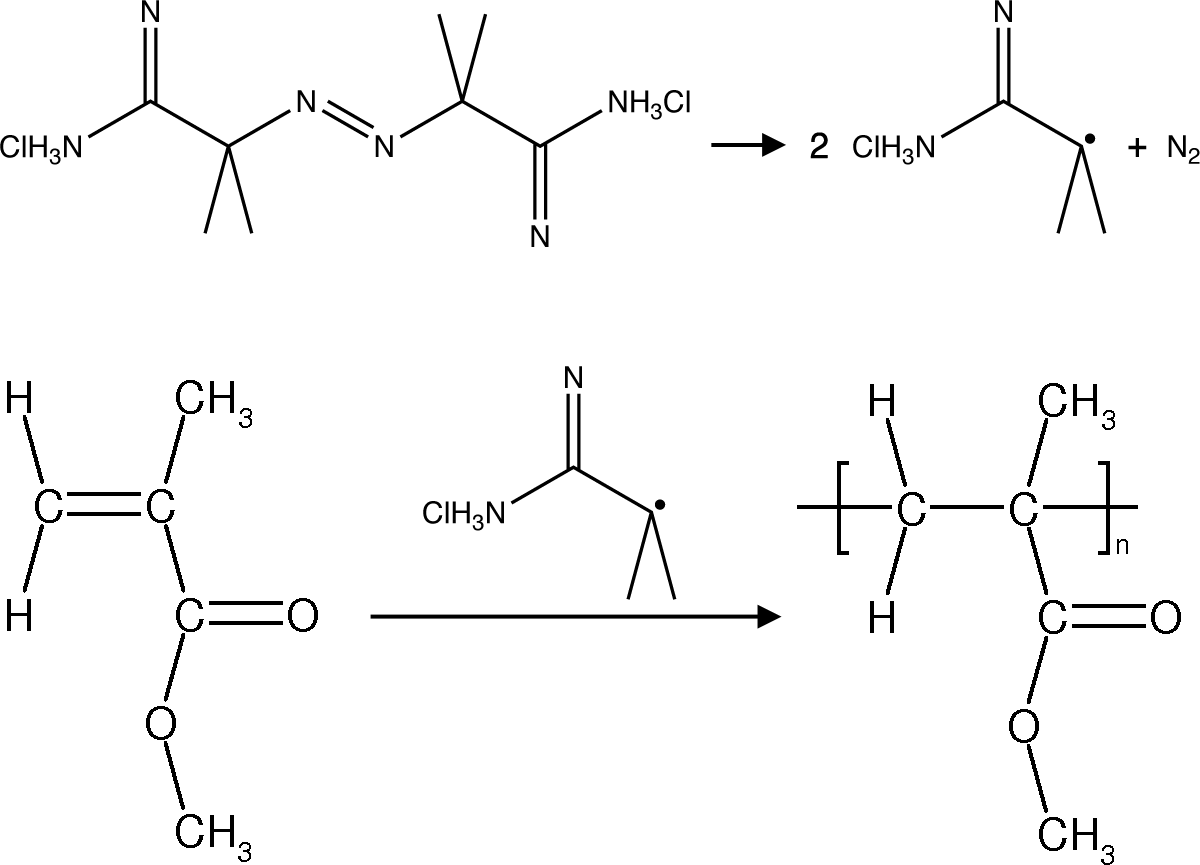
The small uniform diameter particles should appear irridescent if they are close-packed and their size is similar to the wavelength of visible light. The size of the polymethylmethacrylate spheres can be characterized using the settling velocity and Stoke's Law, using evaporative self-assembly and visible spectroscopy, and direct measurement using scanning electron microscopy. The size can also be measured after using the spheres as a template and creating a silica inverse opal.
| Procedure | Wear eye protection |
Chemical gloves recommended |
 Fumehood required Fumehood required |
Synthesis (Write a purpose and method for the synthesis before starting. What are the important variables?)
Procedure modified by George Lisensky, Jacob Horger, and Jiaqi Luo, Beloit
College, from the
Inverse Opal Photonic Crystals Laboratory Guide by R. Schroden and
N. Balakrishnan, University of Minnesota MRSEC, 2001.
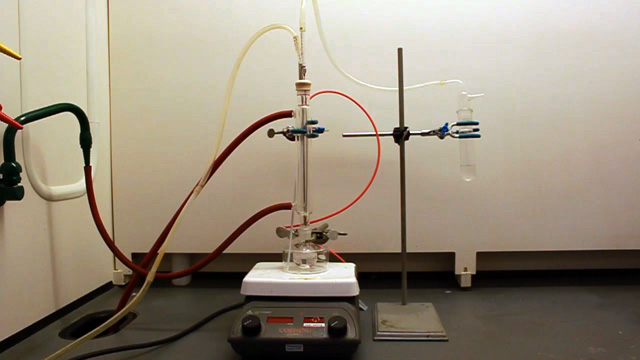
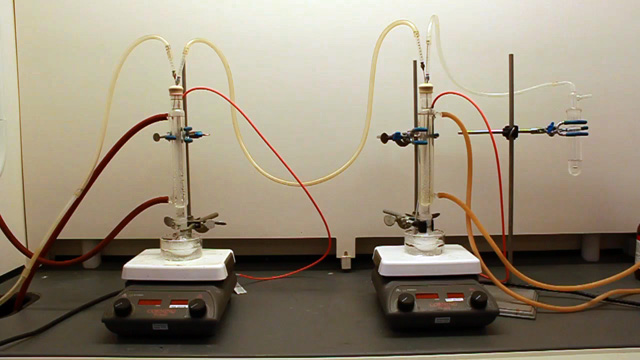
(Not using a teflon sleeve usually results in a permanently polymethylmethacrylate fused joint at the end of the experiment.) Cap the top of the condenser with a septum. Insert a long needle through the septum to deliver nitrogen to the bottom of the condenser. Also insert a short needle through the septum for the nitrogen exit. Connect tubing to the needles. Add a 20x10 mm oval-shaped magnetic stir bar to a 25 mL round bottom flask.
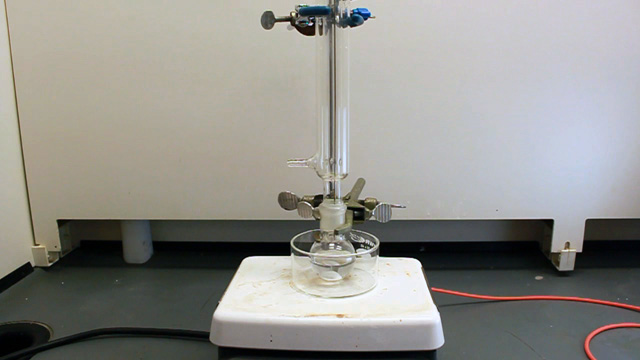
(It is important to use a stir bar designed for a round bottom flask for maximum stirring.) Put a crystallizing dish on a stirrer-hotplate. Clamp the 25 mL round bottom flask in the dish. Add 16 mL pure water and a loosely-clamped condenser assembly.
Connect the water lines to the condenser (in at the bottom and out at the top). Put water in the crystallizing dish and heat to 70 °C. (An automatic temperature controller may be available. The crystallizing dish containing water helps minimize temperature fluctuations and provides a place to measure the temperature.) Spin the stir bar at the maximum speed that works. What is your stir speed?
 Click image for larger view
Click image for larger viewProduct without nitrogen
 Click image for larger view
Click image for larger viewPoorly stirred product
 Click image for larger view
Click image for larger viewWell stirred product
After 40 minutes of heating, remove the condenser. There should not be a noticeable odor if the polymerization was successful. Be sure to return the Teflon joint sleeve.
After the synthesis (same lab period)
- Use the original solution to set up the Stokes Law measurements.
- Add 500µL of original solution to 20 mL water in a large centrifuge tube. Save the capped tube to begin diffraction and SEM experiments next week.
- Use the original solution to fill four labeled microcentrifuge tubes. Save the capped tubes to begin inverse opal synthesis experiments next week. Ideally these tubes will be centrifuged at 2000 rpm for several hours before the next lab period.
- Store any remaining original solution in a capped appropriate-size centrifuge tube for insurance.
- Glassware can be cleaned with acetone since acetone dissolves the starting material and the product.
Stoke's Law Determination of Particle Size (Write a purpose and method for the Stoke's Law determination before starting.)
Procedure developed by George Lisensky and Jiaqi Luo, Beloit College.
Either print each photograph bigger than life on a full sheet of paper and use a ruler or use ImageJ for measuring lengths in digital images. (Use the straight line tool to drag the length of the black tape and menu Analyze/Set Scale to tell the program the actual length of the tape, often 19mm. You can then use the straight line tool to measure lengths in real units.) Be sure all measured data is recorded in your laboratory notebook.
Calculations and Graphs
The beads will fall at the terminal velocity, which depends on their size and the known viscoscity and densities.
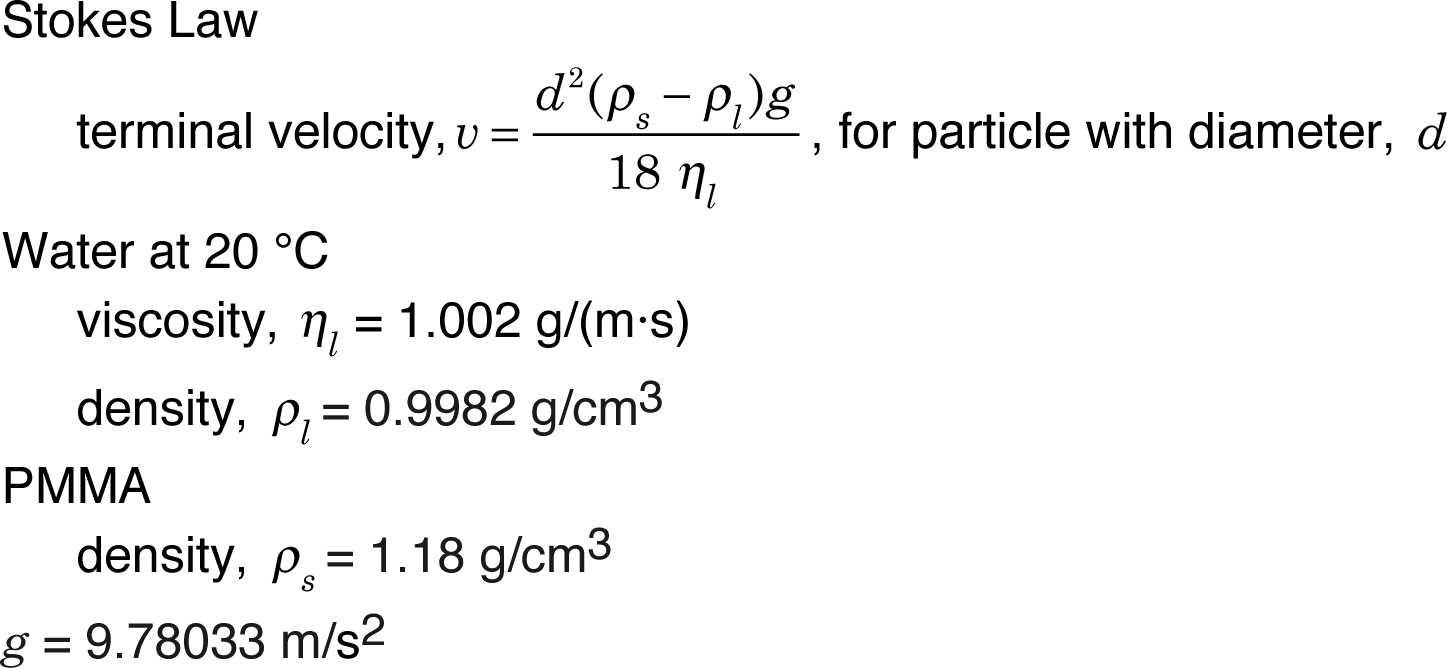
Update your data table at least weekly. For each data point (total time in seconds, distance fallen in mm), calculate the particle diameter. Is the calculated diameter consistent?
Near the end of the experiment plot the distance fallen in mm as a function of the number of seconds and use the slope of the linear portion as the best fit velocity. What is the overall velocity in nm/s? What is the particle size based on this velocity? What does it mean if your y-intercept is not zero?
Optical Diffraction Determination of Particle Size (Write a purpose and method for the diffraction experiment before starting. What are the important variables?)
Procedure by William Schreiter, Richard Amankwah and Karen Nordell, Chemistry Department, Lawrence University.
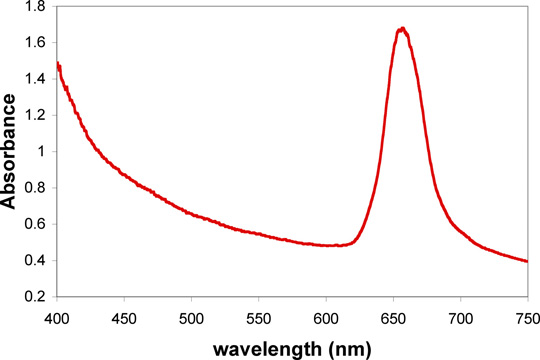
Evaporation of a dilute suspension of polymer nanospheres creates a close packed layer on the surface of a glass slide suspended in the solution. The particle size can be calculated from the visible absorption maximum of this colloidal crystal.
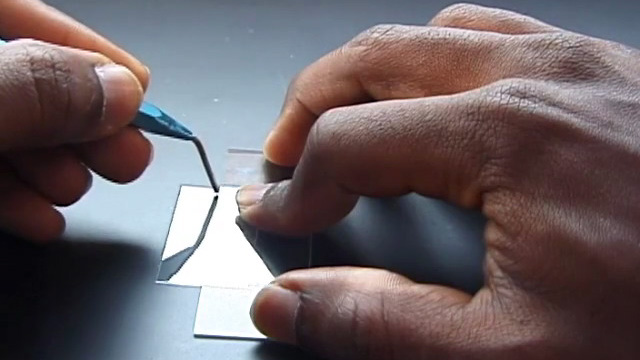
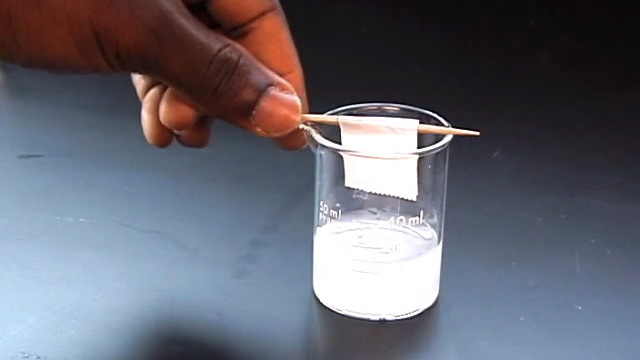
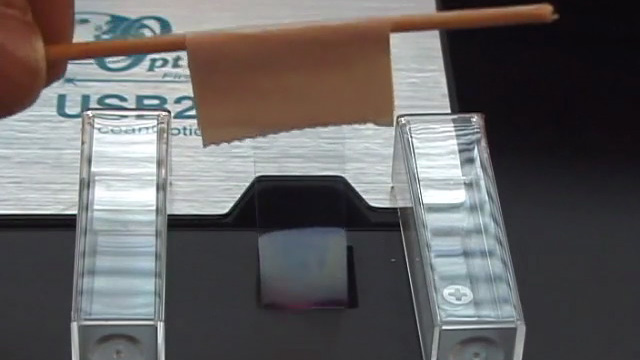
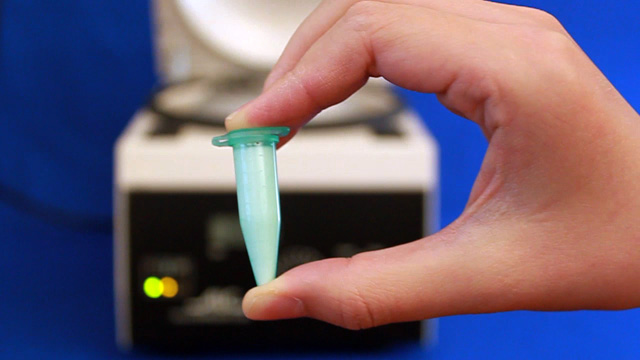
To prepare a sample for SEM or optical diffraction, dilute 500 microliters of previously prepared PMMA nanosphere solution in 20 mL distilled water. (You probably did this step right after the synthesis.) Using a diamond pencil, score and break lengthwise a large glass cover slip (22 mm x 40 mm). The piece of glass should then be able to fit into the sample compartment for visible spectroscopy measurements. Gently attach one of the glass pieces to a toothpick using tape. Write your name on the tape. Clean the glass by soaking in isopropanol for 10 minutes followed by soaking in pure water for 5 minutes. Suspend the glass slide into the diluted PMMA nanosphere suspension you prepared earlier. If desired, more than one glass slide can be suspended in each solution as long as they do not touch each other. Click image for larger view
Click image for larger view25 watt incandescent lamp
Observe the color of the film under reflected light conditions by holding the slide under a bright light source. Observe the color of light transmitted through the film by holding the glass slide up and looking at a bright light source through the slide. How are the colors of reflected and transmitted light related? Obtain the visible absorption spectrum when the glass slide is perpendicular to the beam. If an obvious peak is not seen, repeat the evaporation step and try again.
Use a modified Bragg equation to calculate the size of the nanoparticles from the wavelength of the absorbance peak, λp. The index of refraction is 1.49 for PMMA and 1.00 for air.
λp = the wavelength at maximum absorbance (in nm)
Ds = the diameter of the close packed spheres (in nm)
ns = the index of refraction for the spheres
nv = the index of refraction for the void spaces (presumably air in a dry film)
f = the filling fraction for a close packed structure (0.76)
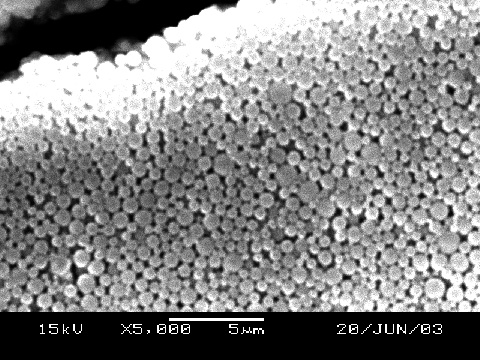
SEM Determination of Particle Size (uses sample from previous step)
Use a diamond pencil to score and then break off a small portion of the diffracted cover glass. Gold coat and examine by SEM with 10,000x. Are the spheres close-packed? What size are the spheres? Sphere size is better measured using multiple spheres in a line and then dividing by the number of spheres measured. Does this size agree with your diffraction results? (ImageJ is useful for measuring lengths. Use the straight line tool to drag the length of the scale bar and menu Analyze/Set Scale to tell the program the actual length of the scale bar. You can then use the straight line tool to measure lengths in real units.)Synthesis of Inverse Opal Photonic Crystals (Write a purpose and method for the synthesis before starting.)
Procedure modified by George Lisensky, Jacob Horger and Jiaqi Luo, Beloit
College, from the
Inverse Opal Photonic Crystals Laboratory Guide by R. Schroden and
N. Balakrishnan, University of Minnesota MRSEC, 2001.
A sol-gel synthesis using tetraethylorthosilicate and close-packed polymethylmethacrylate spheres as a template yields a dimensionally ordered porous silica solid.
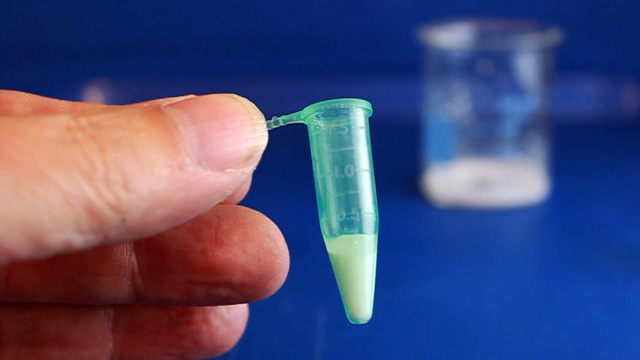
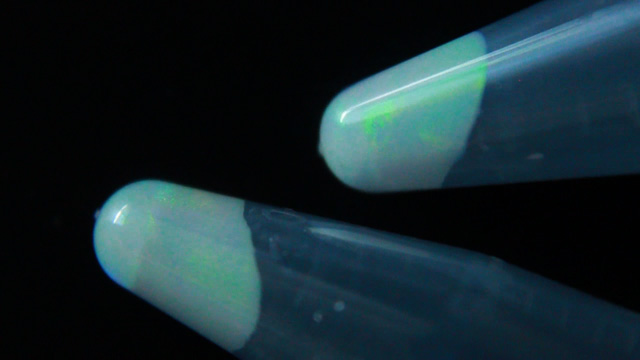
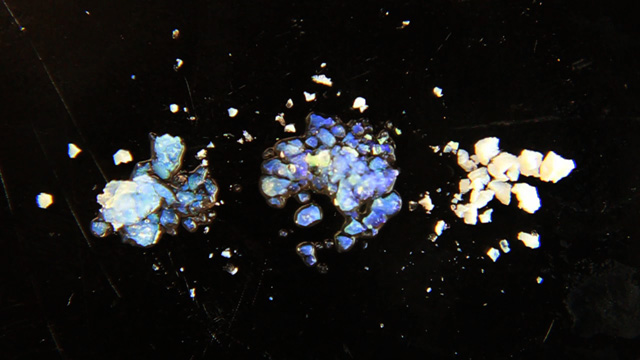
Transfer a portion of your sample to four 1.7 mL microcentrifuge tubes. Always balance the centrifuge by pairing tubes such that opposing tubes have the same amount of solution. Spin your sample at 2000 rpm for several hours. (Spinning at 5000 rpm does not pack as well and spinning at 10,000 rpm will crush the product.) It is desirable to spin the samples before the lab period starts or the experiment will take an extra period. Remove and discard the water above the polymerized polymethylmethacrylate spheres. Caution: Squeeze the dropper before immersing in the water, lower the dropper to just above the solid, and then release to remove the water. If you squeeze with the dropper in the water you will need to repeat the centrifuge step. Put the water in the organic waste. Successful product will change colors with the angle of observation or lighting; the product should look iridescent. Leave the lid open and store the tube in a rack to dry for week. Dry the sample until it can be dumped from the tube. Pour the solid into a combustion boat. Prepare a well mixed solution of 1.0 mL 100% ethanol, 1.5 mL tetraethylorthosilicate, 1.0 mL 25% hydrochloric acid. (This mixture is enough for many samples but it must be freshly prepared.) Wet the spheres with the solution but once all the spheres are wet stop adding liquid or the product may be too dense. Place the combustion boats into the quartz liner of a tube furnace (or a ventilated box furnace) in a hood. Ramp the temperature at 2 degrees C/minute from room temperature to 300 degrees C to complete the silica formation. Hold at 300 degrees C for 2 hours. Ramp the temperature at 2 degrees C/minute to 550 degrees C to decompose the polymethylmethacrylate spheres. Hold at 550 degrees C overnight (ten hours). Cool the oven to room temperature. High quality samples will be apparent by their opalescence.SEM Determination of Inverse Opal Hole Size (uses sample from previous step)
Stick a portion of your sample to the carbon tape on the SEM holder and add your name to the sample map. Samples will then need to be sputtered since SiO2 is non-conducting. On our instrument (using 25kV with spotsize 27 and z 15mm) it should be possible to see holes at 3000x. Look carefully. Hole size is better measured using multiple holes in a line and then dividing by the number of holes measured. (ImageJ is useful for measuring lengths. Use the straight line tool to drag the length of the scale bar and menu Analyze/Set Scale to tell the program the actual length of the scale bar. You can then use the straight line tool to measure lengths in real units.) Be sure to include details of your analysis in your laboratory notebook.
Inverse Opal Properties (uses sample from previous step)
- What color is your product in air? (The index of refraction of silica is 1.460 and of air is 1.000)
- Add a drop of ethanol to a small portion of your product. What color do you observe? (The index of refraction of ethanol is 1.360)
- Add a drop of toluene to a small portion of your product. What color do you observe? (The index of refraction of toluene is 1.496)
- Based on your SEM data, predict the absorption wavelength for air, ethanol, and toluene. How do you need to modify the equation for closed-packed spheres for the inverse material? How well do your predictions match the observed colors? Explain.
Spectra, Images and Graphs
Your notebook should include your Stoke's Law photographs and graph, optical diffraction spectra, and SEM images of both opal and inverse opal materials.
- Did your methyl methacrylate polymerize? What observations support your answer? Using your chemical knowledge of polar/nonpolar, physical properties, and the polymerization mechanism, explain why solid spheres are expected to be produced.
- Based on your Stoke's Law measurements, what is the velocity (nanometers/second) and the corresponding diameter (nanometers) of the spheres?
- Based on evaporative self-assembly to pack spheres, what is value for the photonic band gap (nanometers or eV) and the corresponding diameter (nanometers) of the spheres? What did you use to obtain a constant temperature? How many times did you try the evaporation?
- Based on the SEM image of the opal, did the spheres close-pack and what is the diameter of the spheres (nanometers)? You should measure the diameter for more than one sphere and more than one image and take an average. Report all measured data.
- Based on the SEM image of the inverse opal, are the holes close-packed and what is the diameter of the holes (nanometers)? You should measure the diameter for more than one hole and more than one image and take an average. Report all measured data.
- Based on your hole diameter, predict the absorption wavelength for air, ethanol, and toluene. (How do you need to modify the equation for closed-packed spheres for the inverse material?) How well do your predictions match the observed colors?
- What size are the nanospheres? Do the different methods give the same results within experimental error?
Materials for Synthesis and Stokes Law
Materials for Diffraction
Materials for Inverse Opal
 Click image for larger view
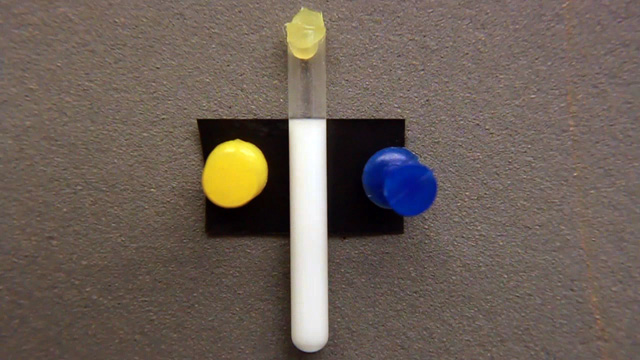
Click image for larger viewUse a tube as shown on right.
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified May 15, 2019 .