HOME > Labs >
Isolation of CdSe Quantum Dot Nanoparticles
Related labs: Synthesis of CdSe Nanoparticles
Related labs: Synthesis of CdSe Nanoparticles

Isolation of CdSe Quantum Dot Nanoparticles
Procedure adapted by Tommy Garting, Lund University, from A.M. Munro et al., Microchim Acta, 160, 345-350 (2008). Luminescence samples of quantum dots resuspended in hexane prepared by Jenny Gilbertson and Tommy Davis.
This procedure separates oleic acid terminated CdSe quantum dot nanoparticles from the octadecene solvent in which they were prepared.
| Procedure | Wear eye protection |
Chemical gloves required |
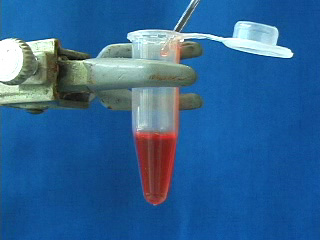
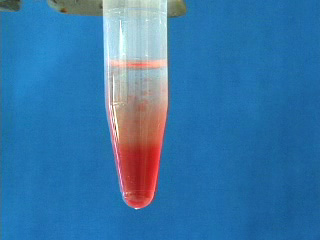
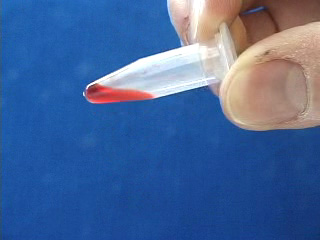
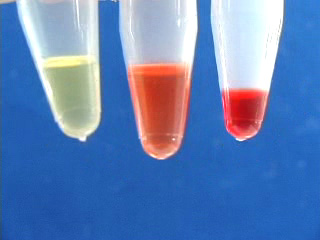
Transfer the octadecene CdSe quantum dot suspension to a micro centrifuge tube. Add 100% ethanol. Cap. Shake to get an emulsion. Add a matched tube and the freshly shaken sample tube to opposite sides of a centrifuge, or use an even number of sample tubes. Spin at 3000 rpm for 5 minutes. Carefully remove the ethanol layer. An oil often appears in the pipet tip. Option: obtain the infrared spectrum of the oil. The removed ethanol solution is cloudy. Add a second ethanol wash. Shake to mix and immediately repeat the centrifuge step. Carefully remove the ethanol. Add a third ethanol wash. When shaking no longer gives a suspension then further centrifugation is not needed. Pour off the ethanol. Different size quantum dots isolated by the same procedure. The quantum dots can be resuspended in an organic solvent (chloroform was used here.) Suspensions of different size quantum dots in an organic solvent (chloroform was used here.) Observe the resuspended quantum dots under long wave UV light (hexane was used as the solvent.)
- What is the identity of the oil that is removed from the quantum dots? How do you know?
- What is the absorption wavelength or color of the quantum dots you isolated?
- How can CdSe exhibit so many different colors?
CAUTION: Avoid physical contact with cadmium selenide since cadmium compounds are carcinogens. If chloroform is used avoid contact or inhalation since it is a likely human carcinogen.
- CdSe quantum dots in octadecene (preparation)
- 100% Ethanol
- Hexane (or chloroform)
- microcentrifuge and tubes
- Pasteur pipets and bulbs
- Vials
- UV light source
Developed in collaboration with the
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified June 16, 2013 .
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified June 16, 2013 .