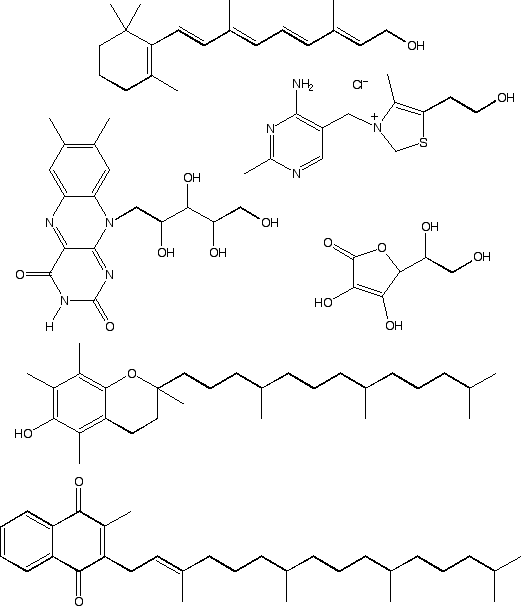
Are Vitamins Polar or Nonpolar?
Based on the formula, predict whether each of these molecules is polar or non-polar and explain your reasoning.
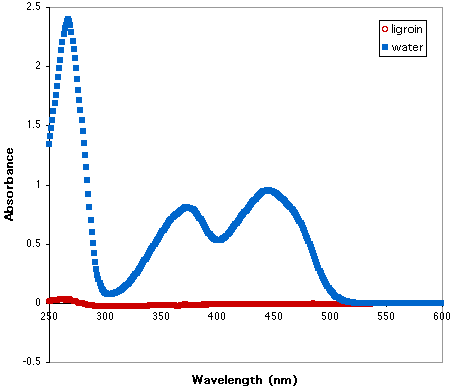
The Laboratory Experiment in Exploration 3A asks you to work together in groups to collect UV spectra of vitamins in water and ligroin (an organic solvent). A vitamin sample is placed in 2 mL of water and 2 mL of ligroin, the layers are shaken, and the vitamin partitions between the solvents. The solution is then allowed to separate into two layers and a spectrum is obtained for each layer. Which layer is which? How do you know? What can you infer from your observations about the density of water vs. ligroin?
Absorbance and wavelength data for individual vitamins:
A, B1,
B2, C, E, K
(Spectra obtained by Chia Goh and George Lisensky at Beloit College.)
Click on the figure below to open an Excel file containing the data and spectra for each vitamin stored in a separate sheet. (If your browser does not have Excel configured as a helper application, hold the mouse button down over the link. Drag down to "Save this Link as..." in Netscape or "Download Link to Disk" in Explorer. Open the saved file in Excel.) You will notice that in these figures the red open circles indicate the absorbance in the organic solvent while the square blue solids indicate the absorbance in the water layer. Which vitamins are more soluble in the ligroin? Which are more soluble in the water? Do these results fit with your predictions?
Vitamin Spectra | Fuss about Fats | ChemConnections