Electrochromic Prussian Blue Thin Films
Modified by Jason Marmon and George Lisensky from J. J. Garcia-Jareño, D. Benito, J. Navarro-Laboulais, and F. Vicente, "Electrochemical Behavior of Electrodeposited Prussian Blue Films on ITO Electrodes," J. Chem. Educ., 75, 881-884 (1998).
Prussian Blue, Fe(III)4[Fe(II)(CN)6]3.xH2O (Click for jmol), is strongly colored and has been made by chemists for 300 hundred years. For more information on
its history, see C&E News, 83(18), 32-35 (2005) or Pigments through the Ages.
In this experiment [Fe(III)(CN)6]-3 is electrochemically reduced on a glass electrode to produce [Fe(II)(CN)6]-4.
The [Fe(II)(CN)6]-4 formed at the electrode reacts with Fe(III)Cl3 in solution to give insoluble Prussian Blue on the electrode.
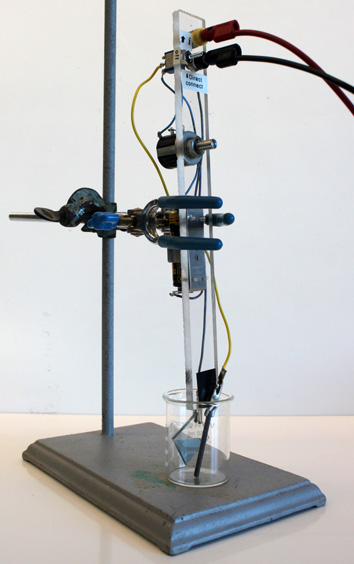
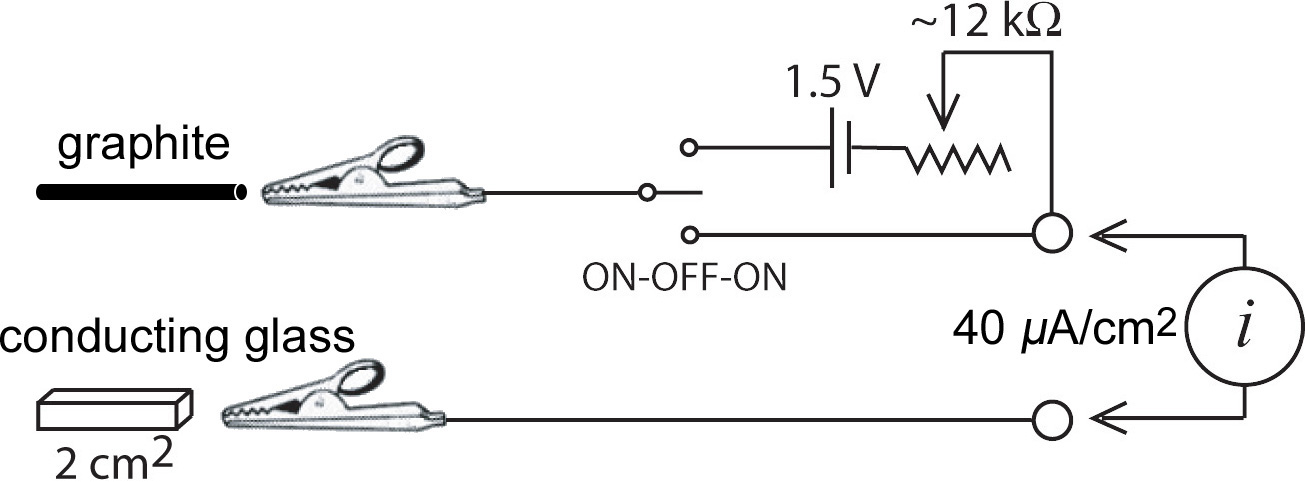
You will use an electrical circuit (see diagram below) to supply current for a known number of seconds. Knowing the current, the amount of time, and the electrode area lets you calculate the coating thickness.
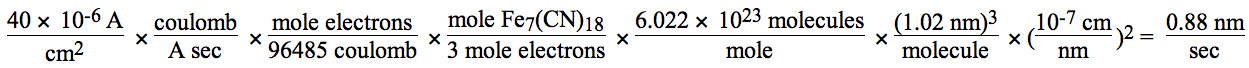
What is the minimum thickness of Prussian Blue that can be seen by eye?
Does the coating on the glass change color with applied voltage, i.e., does a 200 nm thick layer of Prussian Blue exhibit electrochromism? Electrochromic displays, where an electrochemical redox reaction changes the pixel state, do not need constant application of power. Such pixels are useful for electronic books where high contrast and low power consumption are important and for smart windows to reduce energy use.
| Procedure | Wear eye protection |
Obtain two cables, a multimeter, and a tin oxide-coated glass square. Set the multimeter to measure resistance (Ω). What reading do you get when the cables are touching? What reading do you get when the cables are not touching? (The latter is an open circuit with a very large resistance.) Identify the conducting side of the glass square. The conducting side will have a resistance of 20-30 ohms and the non-conducting side will have a very large resistance. Obtain a battery and circuit, clamp and ringstand, a graphite electrode, and a 50 mL beaker.
After you have determined the minimum coating thickness that can be seen, continue the electrolysis for a total of 200-300 seconds to prepare a relatively thick film. Record the total seconds that current was applied to the sample. Rinse the electrode with water. Place the rinsed electrodes in 25 mL 1.0 M KCl, keeping the clip above the solution. • Remove the battery and the multimeter from the circuit.
• Move the toggle switch to the direct connect position so the cables connect directly to the electrodes in solution.
• Connect the glass electrode to battery (–) and the graphite electrode connected to battery (+). Does the color change?
• Touch the two cables together, i.e., complete the circuit with no battery. Does the color change?
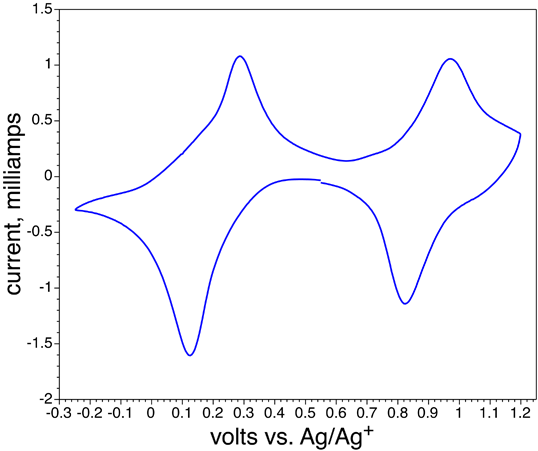
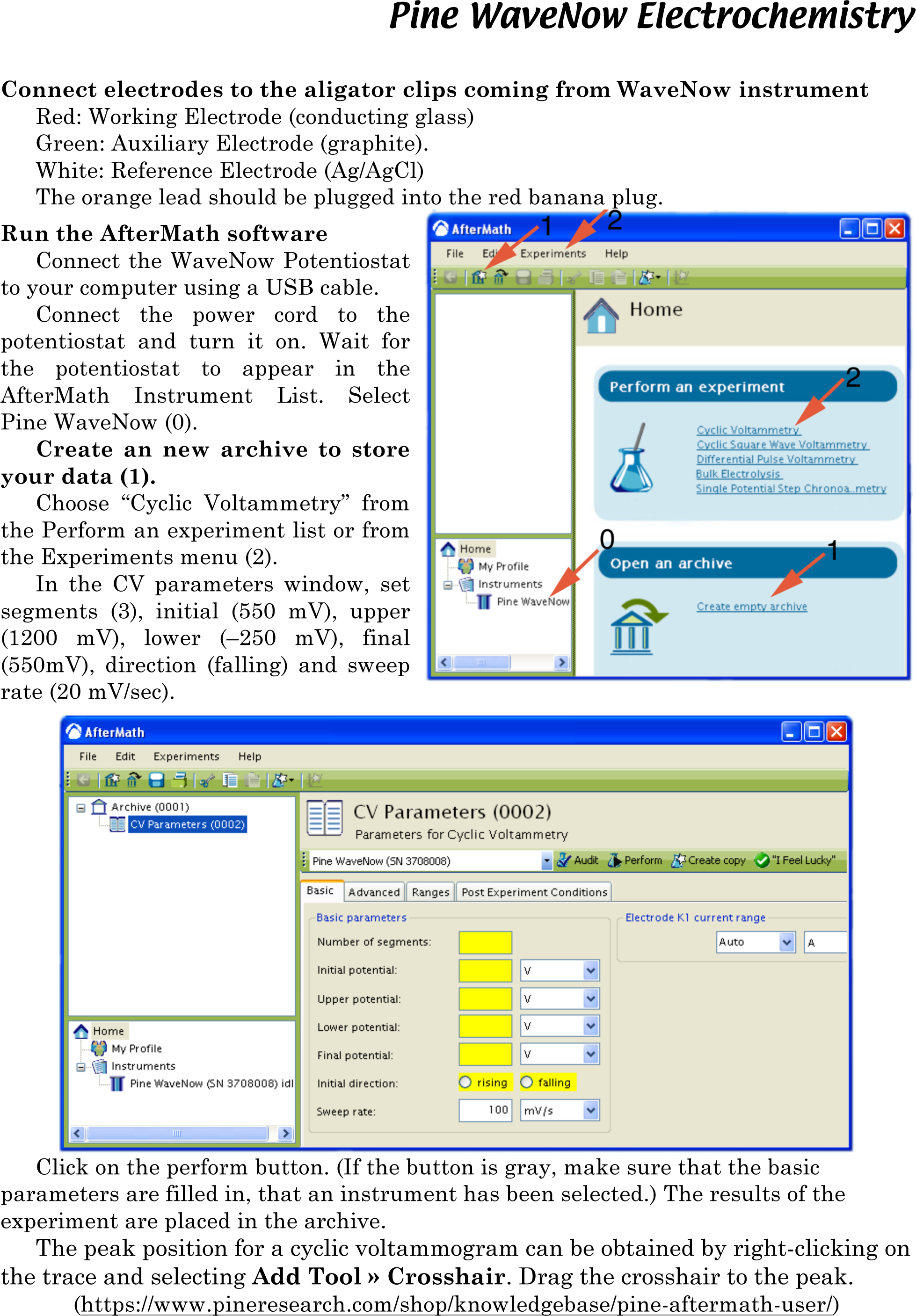
• Repeat the previous two steps several times. (Repetition is thought to make the film more stable for the next part.) Record the cylic voltammogram using the Prussian Blue coated glass as the working electrode, the graphite as the auxiliary electrode, and a Ag/AgCl electrode as the reference electrode. All three electrodes must be in the beaker. Scan at 20 mV/sec from +550 mV to -250 mV to +1200 mV to +550 mV. See directions below. When the voltage matches a redox reaction the current increases. Does the color change at the same time? Repeat the scan as necessary to observe the color changes.
Conclusions
- How thin is the minimum coating you can see? How thick is the thickest film you made? Show your calculations.
- What color is the reduction product of Prussian Blue? What is the redox potential for this reaction?
Fe(III)4[Fe(II)(CN)6]3 + 4 K+ + 4 e- → K4Fe(II)4[Fe(II)(CN)6]3(Take the average of your observed forward and reverse peak voltages.)
- What color is the oxidation product of Prussian Blue? What is the redox potential for this reaction?
Fe(III)4[Fe(II)(CN)6]3 + 3 Cl- → Fe(III)4[Fe(III)(CN)6]3Cl3 + 3 e-(Take the average of your observed forward and reverse peak voltages.)
- What is the source of the K+ and Cl- ions in these redox reactions?
- Consider smart window applications for electrochromic materials. How well does this work on the Boeing 787 Dreamliner windows?
 Click image for larger view
Click image for larger view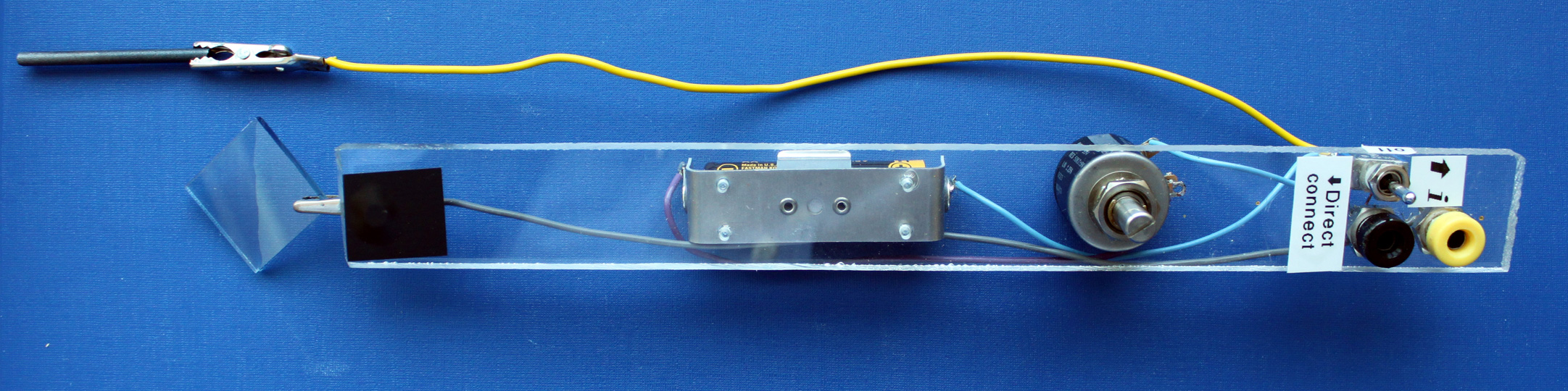 Click image for larger view
Click image for larger viewPlexiglass strip (1/4" x 1" x 9.5") from local hardware store or McMaster-Carr Clear Acrylic Sheet 8589K81. Drill holes to match electronic parts.
Parts list from http://www.mouser.com
Battery Holder, Mouser 534-139
50 k-ohm 10 turn Potentiometer, Mouser 594-53411-503
Banana Jacks, Mouser 530-108-0907-1
Aligator Clips, Mouser 548-30-BL
ON-OFF-ON through hole Toggle Switch, Mouser 633-M201303
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified January 22, 2020 .