Electrochromic Prussian Blue Thin Films
Modified by Jason Marmon and George Lisensky from J. J. Garcia-Jareño, D. Benito, J. Navarro-Laboulais, and F. Vicente, "Electrochemical Behavior of Electrodeposited Prussian Blue Films on ITO Electrodes," J. Chem. Educ., 75, 881-884 (1998).
In this experiment K3[Fe(III)(CN)6] is electrochemically reduced at a glass electrode to produce K4[Fe(II)(CN)6]. The K4[Fe(II)(CN)6] at the electrode reacts with Fe(III)Cl3 in solution to give insoluble Prussian Blue, Fe(III)4[Fe(II)(CN)6]3, on the electrode. The approximately 100 nm thick layer exhibits visible electrochromism, i.e., the coating on the glass changes color with applied voltage. (Prussian Blue has been made by chemists for 300 hundred years. For more information on its history, see http://webexhibits.org/pigments/indiv/overview/prussblue.html.)
| Procedure | Wear eye protection |
(The sixth term in the equation above comes from the cubic unit cell dimensions. Inorg. Chem., 16(11), 2704-2710, (1977)).
Changing the applied voltage (next step) will change the color of the thin film.
- Glass electrode connected to battery (–) and platinum or graphite electrode connected to battery (+); negative voltage applied.
- Glass electrode directly connected to platinum or graphite electrode;
zero volts applied.
Films are reported to be more stable if you repeat steps 1 and 2 several times before going on. - Glass electrode connected to battery (+) and platinum or graphite electrode connected to battery (–); positive voltage applied.
- Glass electrode directly connected to platinum or graphite electrode; zero volts applied.
- Repeat.
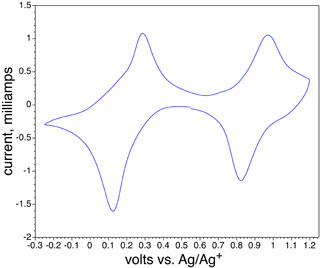
What color is the reduction product? What is the redox potential for the reaction?
Fe(III)4[Fe(II)(CN)6]3 + 4 K+ + 4 e- = K4Fe(II)4[Fe(II)(CN)6]3
What color is the oxidation product? What is the redox potential for the reaction?
Fe(III)4[Fe(II)(CN)6]3 + 3 Cl- = Fe(III)4[Fe(III)(CN)6]3Cl3 + 3 e-
What is the source of the K+ and Cl- ions in the redox reactions?
- 0.05 M HCl (Each preparation needs 5 mL. Dilute 5 mL conc HCl to 250 mL with water.)
- 0.05 M K3[Fe(CN)6] (Each preparation needs 10 mL. Dissolve 1.65 g in 100 mL water.)
- 0.05 M FeCl3.6H2O (Each preparation needs 10 mL. Dissolve 1.35 g in 100 mL water with a drop of HCl.)
- 1.0 M KCl (Each preparation needs 25 mL. Dissolve 18.6 g in 250 mL water with two drops of HCl to lower the pH.)
Equipment
- 50 mL beaker
- Conductive Glass (1" x 1" x 2.3mm TEC 15 glass) Hartford Glass Co, 735 E Water Street, Hartford City, IN 47348 Phone: 765-348-1282. Glass can be reused by removing prussian blue coatings with concentrated ammonia (solubility increases with pH).
- Ohmeter
- Pt wire or graphite electrode
- 1.5 Volt battery, holder, and 50 K ohm variable resistor for controlling deposition current. See electrical diagram.
- Electrochemical apparatus for obtaining cyclic voltammograms
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified July 10, 2019 .