Synthesis of Aqueous Ferrofluid
Procedure modified by Jonathan Breitzer and George Lisensky from "Preparation and Properties of an Aqueous Ferrofluid" by Patricia Berger, Nicholas B. Adelman, Katie J. Beckman, Dean J. Campbell, Arthur B. Ellis, and George C. Lisensky, J. Chem. Educ., 76, 943-948 (1999).
Ferrofluids are colloidal suspensions of magnetic nanoparticles. Ferrofluids respond to an external magnetic field enabling the solution's location to be controlled through the application of a magnetic field. Fe3O4 magnetite nanoparticles can be produced by mixing Fe(II) and Fe(III) salts together in a basic solution. The particles must remain small and separated from one another in order to remain suspended in the liquid medium. In the procedure below, what is added to prevent the nanoparticles from approaching one another too closely? Once prepared, ferrofluids have the captivating property of exhibiting "spikes" when placed in the proximity of a strong magnet. Magnetic nanoparticles are used in medicine for tag-and-drag analyses. See the graphical abstract in ACS Omega 2023, 8, 24, 21745-21754 for a recent example.
| Procedure | Ferrofluids can be messy. The particular ferrofluid you will prepare will permanently stain almost any fabric. |
1 M FeCl3 in 2 M HCl (shown at left)
2 M FeCl2 in 2 M HCl (shown at right) Add 4.0 mL of 1M FeCl3 and 1.0 mL of 2M FeCl2 solution to a 100 or 150 mL beaker. Continue stirring with a glass rod throughout the dropwise addition of 50 mL 1.0 M aqueous NH3 solution over a period of about 5-10 minutes, adding approximately 1 mL every 10 seconds. Avoid addition that is faster than the solution can be mixed, but also avoid addition that is so slow that the particles grow large. How long did your addition take? CAUTION: Although 1 M NH3 is fairly dilute, NH3 is a strong base. Let the magnetite settle. You can speed the settling process by putting a magnet under the container. Decant (pour off) and discard the clear liquid without losing a substantial amount of solid. This works best if you keep a magnet under the container. Transfer the solid to a weighing boat with the aid of a few squirts from a wash bottle. Use a strong magnet to attract the ferrofluid to the bottom of the weighing boat. Pour off and discard as much clear liquid as possible, again keeping the magnet under the weighing boat. Rinse with water from a wash bottle and decant the rinse as before. Remove the magnet. Add 1-2 mL of 25% tetramethylammonium hydroxide. Gently stir with a glass rod for at least a minute to suspend the solid in the liquid. Use a strong magnet to attract the ferrofluid to the bottom of the weighing boat. Pour off and discard the dark liquid. Move the strong magnet around and again pour off any liquid. If the ferrofluid does not spike, continue to move the strong magnet around, pouring off any liquid. What happens when you move a magnet under the ferrofluid? If you are using a very strong magnet you might get more interesting results by varying the distance of the magnet below the boat.
XRD (option and calculations)
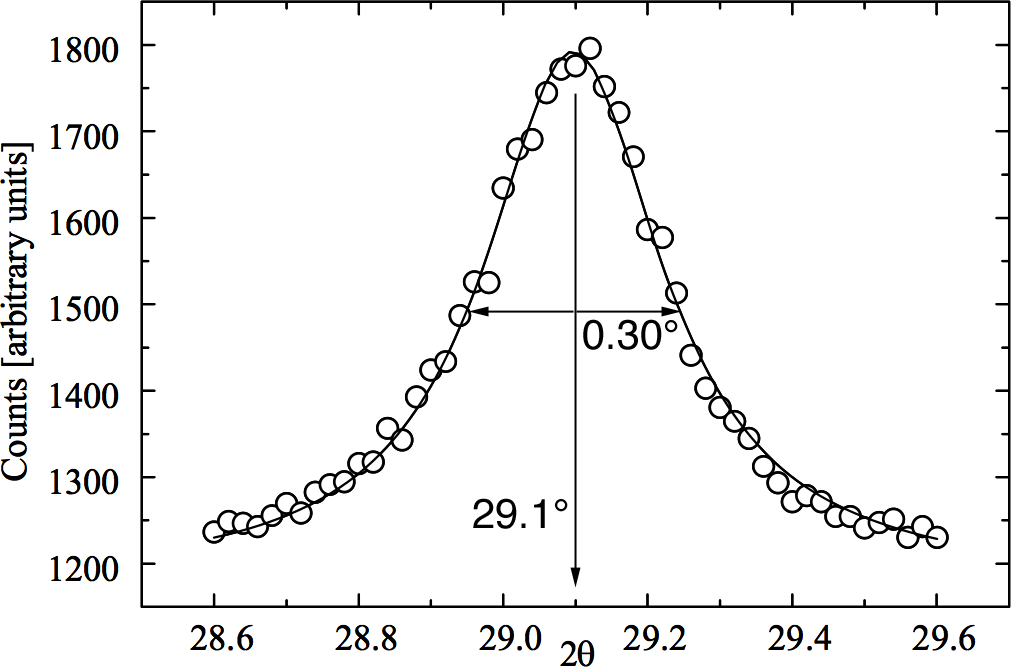
Diffraction peaks are a result of constructive interference of X-rays reflected by crystal planes. The more planes the sharper the peak so the smaller the crystallite size the broader the diffraction peak.
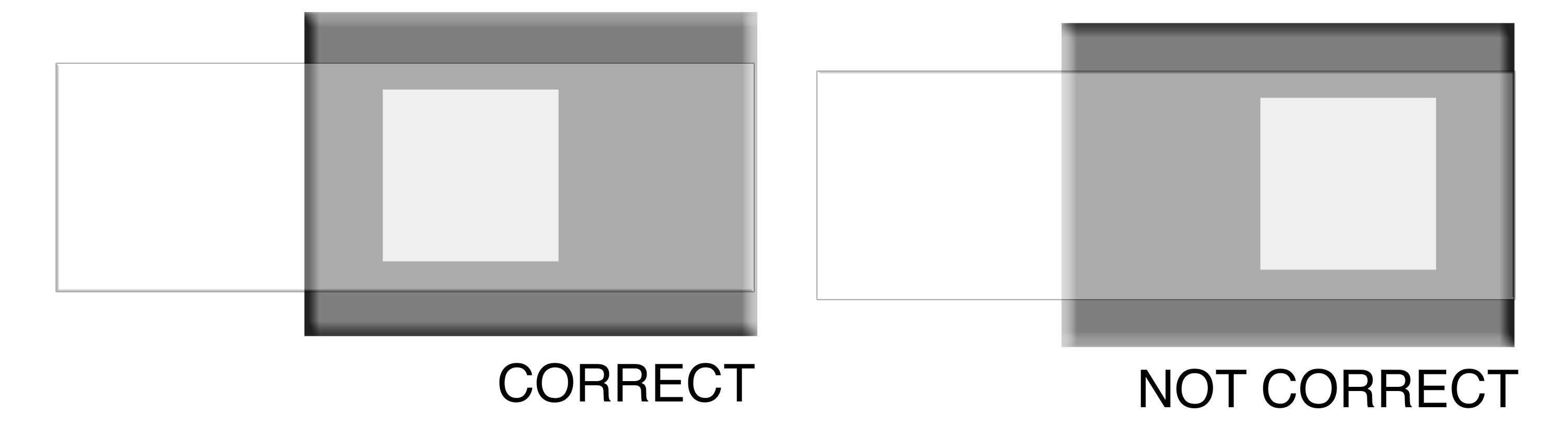
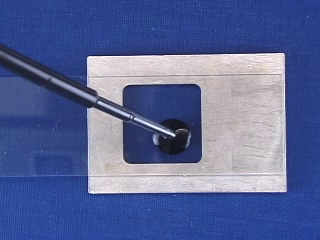
Transfer your ferrofluid to the correct position on a glass slide (the aluminum holder is placed under the slide as a template.) When the solution has dried, obtain the powder x-ray diffraction spectrum (2θ = 25-65°). Measure the peak width for several peaks as shown at right. Estimate the particle diameter
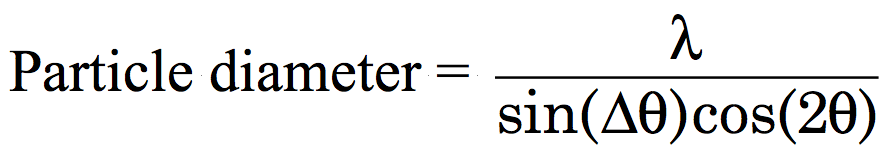
To analyze the XRD data, open the MDI Jade program.
 Select the file you previously created. The dog icon at the upper left allows you to fetch the most recent scan or you can use the folder icon to find files.
Select the file you previously created. The dog icon at the upper left allows you to fetch the most recent scan or you can use the folder icon to find files.
 Click on the icon with the vertical lines to find the peaks and display the d values. To get peak widths for calculating particle size, left click on the double parabola icon to fit a parabola; then right click on the same icon to list the FWHM. Print the window and use the four or five tallest peaks for your analysis.
Click on the icon with the vertical lines to find the peaks and display the d values. To get peak widths for calculating particle size, left click on the double parabola icon to fit a parabola; then right click on the same icon to list the FWHM. Print the window and use the four or five tallest peaks for your analysis.
- Were you able to prepare ferrofluid and observe spiking in the presence of a magnet?
- If you did not observe spiking with your sample, you will need to inspect a sample that did spike. Pick one spike in the middle. How many spikes were immediately around that spike and how were they arranged? You might draw a picture viewed from above.
- Examine the starting FeCl2 and FeCl3 solids used to prepare magnetite (leave the solids in their sealed containers.) How do they respond to a magnet? Which is magnetic?
- Draw the Lewis dot structure of tetramethyl ammonium hydroxide. What is the purpose of adding this chemical? Explain chemically how tetramethyl ammonium hydroxide works better than ammonium hydroxide to keep the nanoparticles suspended.
- What evidence do you have for the formation of nanoparticles?

 Click image for larger view
Click image for larger view Click image for larger view
Click image for larger view
-
Stock solutions for 50 preparations
- 2 M HCl (21 mL conc HCl in 250 mL water) for making iron solutions.
- 2.0 M FeCl2(H2O)4 in 2 M HCl. (Each student
needs 1 mL. Dissolve 19.9 g in 50 mL 2 M HCl. Verify color as you weigh out the solid. This material dissolves
readily but the solution reacts with oxygen and should be freshly prepared.)
Orion Pearce reports that partially oxidized FeCl2(H2O)4 solid can be purified by rinsing with a minimal amount of 2 M HCl, either by swirling the solid with HCl in a beaker and decanting the brown liquid or using a short pipet column with a cotton plug. The resulting green solid does not save since the residual HCl accelerates the oxidation process, but the rinsed solid does work for the ferrofluid synthesis. - 1.0 M FeCl3(H2O)6 in 2 M HCl (Each student needs 4 mL. Dissolve 54.1 g in 200 mL 2 M HCl. This material often dissolves slowly but the solution is air stable and can be prepared in advance.)
- 1.0 M NH3 in water. (Each student needs 50 mL. Dilute at least 200 mL of concentrated ammonium hydroxide with water to 3.0 L. It may be possible to use household ammonia; its concentration varies and an initial estimate would be 1.0 L household ammonia diluted to 3.0 L.) Open containers of ammonia will smell bad and their concentration will decrease leading to poor results.
- 25% tetramethylammonium hydroxide in water (commercially available from Aldrich, Fisher, etc.). A strong, fishy, amine odor indicates hydrolysis products which may interfere with the synthesis. In this case adding an additional 1-2 mL may help.
- A way to measure iron solutions (one buret dispensing each iron solution works well; individual pipets could also be used)
- 100 or 150 mL beakers
- A way to slowly add the ammonia solution (a buret or separatory funnel or eyedropper)
- Glass rods for stirring
- Plastic weighing boats
- Wash bottles
- Disposable gloves
- Ferrite ceramic magnets from Bluefeather or CMS-Magnetics
- Option: Cow magnets
- Option: XRD spectrometer and glass slides
Equipment
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified April 25, 2022.