Synthesis of Nanocrystalline Y2O3:Eu Phosphor
Procedure based on D. B. Bolstad and A. L. Diaz, J. Chem. Educ., 79, 1101-1104 (2002).
A small percentage of cations in an inert Y2O3 host are replaced by luminescent Eu+3 ions to give a red phosphor. The quality of x-ray diffraction depends on the particle size and the efficiency of the 4f phosphor transitions is also influenced by crystallinity of the host. In this experiment measurements are made on the initial product and on the product after further heating. The study of solid-state luminescence impacts a wide variety of technologies, including display (CRTs and flat televisions), lighting (fluorescent lamps and mercury-free lamps), and medical imaging.
| Procedure | Wear eye protection |
Chemical gloves recommended |
 Fumehood recommended |
Thermal gloves recommended |
Add 3 mL water, 1.00 g Y(NO3)3.6H2O, 0.06 g Eu(NO3)3.6H2O, and 0.41 g urea to a 30 mL beaker. Because a potentially explosive mixture is being prepared, add the chemicals to water rather than diluting a mixture of the solids.
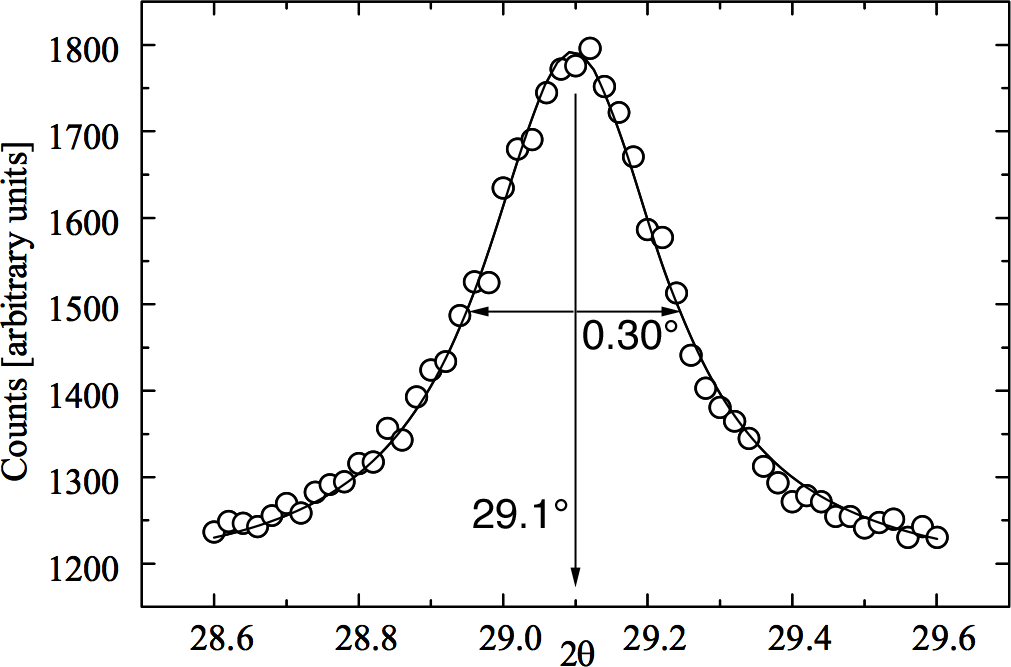
Cover with a watch glass. Heat the solution in a hood in an oven at 500 degrees C for 10 minutes (long enough for the reaction to finish and the brown NO2 gas to disperse). The heating should be done in a hood in an oven with the door closed. The movie shows what you would observe if you could see into the oven. Caution: the brown NO2 gas produced by the reaction is extremely hazardous to inhale. The mixture reacts to form a foamy white solid. Transfer half the product to an alumina crucible. Heat to 850 degrees C overnight. Alternate illumination by room light and short-wave ultraviolet light. The sample on the left has not had the final heat treatment. Prepare samples for powder diffraction by spreading the powder on double-sided tape on a glass microscope slide. Compare the powder x-ray diffraction spectrum (2θ = 25-65°) of the of the product before and after the last heating step Measure the peak width for several peaks as shown at right. Diffraction peaks are a result of constructive interference of X-rays reflected by crystal planes. The more planes the sharper the peak so the smaller the crystallite size the broader the diffraction peak. Estimate the particle diameter,
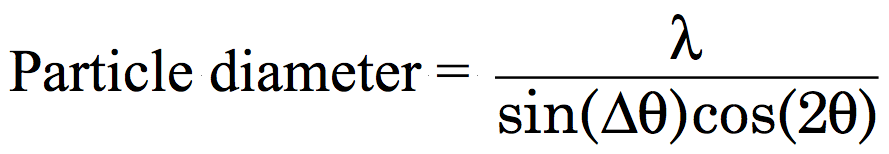 where λ is the X-ray wavelength, Δθ is the peak-width at half-height (FWHM) and 2θ is the peak location. (For even better results use the peak width of the sample minus the peak width of the same peak in a bulk sample as the peak width.)
The X-ray wavelength depends on the target in the instrument X-ray tube. Common targets are Cr (2.28970Å), Fe (1.93604Å), Co (1.78897Å), Cu (1.54056Å), or Mo (0.70930Å) metals.
where λ is the X-ray wavelength, Δθ is the peak-width at half-height (FWHM) and 2θ is the peak location. (For even better results use the peak width of the sample minus the peak width of the same peak in a bulk sample as the peak width.)
The X-ray wavelength depends on the target in the instrument X-ray tube. Common targets are Cr (2.28970Å), Fe (1.93604Å), Co (1.78897Å), Cu (1.54056Å), or Mo (0.70930Å) metals.
- Yttrium(III) nitrate hexahydrate(99.9%), Strem 93-3937 or Aldrich 237957-25G
- Europium(III) nitrate hexahydrate (99.9%), Strem 93-6310 or pentahydrate Aldrich 207918-1G
- Urea
- 30 mL beaker and watch glass
- 500 degree C oven and tongs
- crucible and cover
- 850 degree C oven
- ultraviolet light
- powder diffractometer
Equipment
Developed in collaboration with the
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified May 13, 2019 .
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified May 13, 2019 .