Preparation of Surface Conductive Glass
Procedure modified by D. Keefer, E. Eibergen, A. Nicholas and G. Lisensky from that of J. Tanaka and S. L. Suib, "Surface conductive glass," J. Chem. Educ. 61, 1104 (1984).
Reduction of tin(IV) on a hot surface gives a mixture of SnO and SnO2. The rutile lattice structure of the product can be pictured as that of SnO2 with some oxide ions missing. This non-stoichiometric oxide is a semiconductor. Related coatings are used for flat panel displays.
| Procedure | Wear eye protection |
Chemical gloves recommended |
 Fumehood recommended |
Thermal gloves recommended |
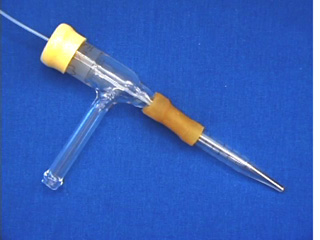
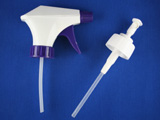
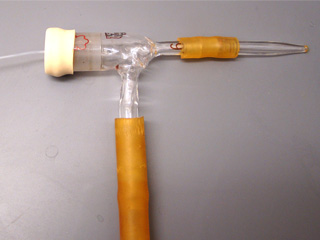
Put a ceramic tile glazed side up in an oven to serve as a liner. Preheat the oven to 600 degrees C. Assemble a concentric nebulizer (left) and connect the sidearm to a compressed gas source. Alternatively obtain a mister (right) that is chemically robust when spraying tin(IV) chloride methanol solutions. Pump hair spray misters for brands that contain alcohol sometimes work. The spray from pump misters covers a wide area and care should be taken with the overspray. Adjust the sprayer. For the concentric nebulizer, slide the capillary tube through the pipet tip. Slowly pull the capillary tube back until solution aspirates. (If bubbles emerge from the other end the capillary tip has been pulled back too far.) Liquid will rise up the tube and eventually spray out the tip. To stop spraying, extend the capillary through the tip. To empty the tube, pull the capillary inside the tip. Test sprayer first with water by spraying a paper towel. Next rinse the sprayer with methanol and then empty. Put glass microscope slides on another ceramic tile and place in the oven. Prepare a solution of 5 g SnCl45H2O dissolved in 5 mL methanol. Do not use anhydrous SnCl4(l) since it reacts violently with methanol. After heating the glass and tile for 10 minutes, remove both from the oven, immediately spray with a fine mist of tin(IV) chloride solution, and return the glass and tile to the oven for 2 minutes to reheat. Repeat the treatment several times as the tin(IV) chloride is reduced by methanol on the hot surface to give a mixture of SnO and SnO2. The rutile lattice structure of the product can be pictured as that of SnO2 with some oxide ions missing. This non-stoichiometric oxide is a semiconductor whose conductivity depends on the quantity of defects. Doping with Sb(III) can act to produce missing oxides in the structure and increase the conductivity. Do resistance measurements for the glass follow Ohms Law? How could you test whether the material is a metal or a semiconductor? How transparent is your conductor?
- SnCl45H2O dissolved in methanol (1 g/mL)
- 600 degree C oven
- ceramic tiles
- microscope slides
- tongs
- Optional: 0.10 g Sb2O3 dissolved in 1 mL conc HCl and then added to 5 mL of the tin(IV) chloride solution.
Use glassblowing to add a T-joint to 14/20 ground glass joint (Ace Glass 7568-06, Chem Glass CG-106-05, or Wilmad-Lab Glass LG-1016-110)
Septum cap to fit the ground glass joint
Thin plastic tubing that connects to the needle and passes through the septum cap
Compressed gas source (the apsirator works by blowing gas through the glass)
Developed in collaboration with the
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified June 16, 2013 .
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified June 16, 2013 .