A Liquid Crystal Sensor
The synthesis procedure shown here was adapted by Katie Cadwell, Steve Ng and Ken Gentry from work by Kun-Lin Yang, Katie Cadwell, and Nicholas Abbott, J. Phys. Chem. B, 108,20180-20186 (2005).
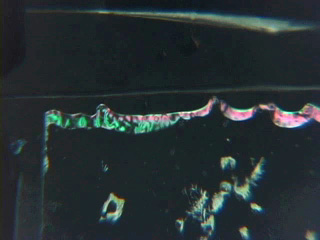
A liquid crystal applied over a dilute copper perchlorate solution dried on a glass slide responds to dimethyl methylphosphonate.
| Procedure | Wear eye protection |
Chemical gloves recommended |
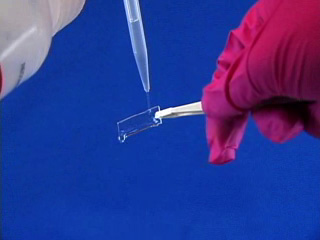
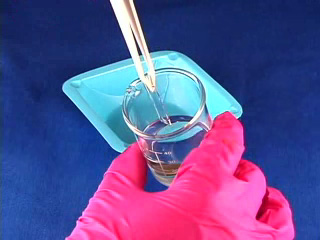
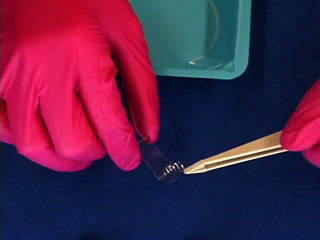
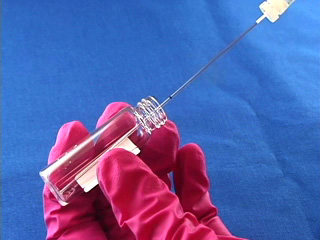
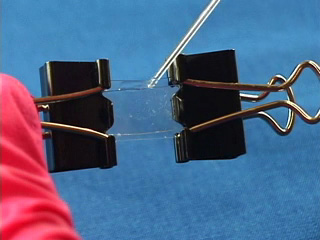
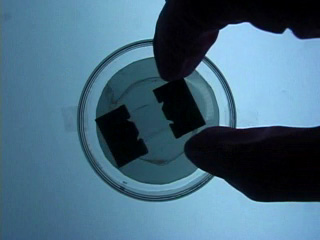
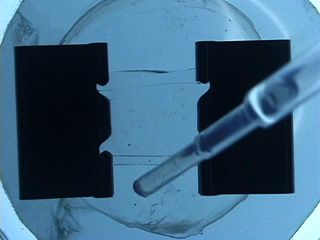
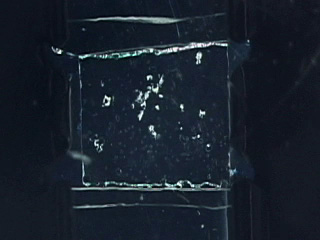
Dissolve 0.18g copper(II) perchlorate in 100 mL ethanol to give a 5 mM solution. Clean the cut glass slides in ethanol. Soak the clean slides in the copper perchlorate solution for several minutes. Let the slides dry without touching them. Cut small pieces of plastic wrap. Sandwich the plastic wrap at both ends between the two slides. Use tweezers and do not touch the center faces of the slides. Clamp both ends of the slides with binder clips. The clips should clamp over the plastic wrap leaving an empty volume between the slides. Load the capillary tube with a small amount of liquid crystal. Warm liquid crystal and a capillary tube with a blow dryer and transfer liquid crystal to the space between plates. Remove handles from binder clips. Obtain a petri dish with a polarizing filter attached to each plate. Put the holder in the petri dish. Rotate the cover to get maximum darkness (polarizers oriented at 90° with respect to each other.) The liquid crystal is in a homeotropic orientation because it is bound to the copper salts on the slides. The liquid crystal will not bend the light in this orientation and the crossed polarizers prevent light from exiting the Petri dish. Add a drop of dimethyl methylphosphonate and immediately replace the cover. A light band will appear at the edges of the sensor. The dimethyl methylphosphonate has bound to the copper salt on the slide, displacing the liquid crystal. The liquid crystal is free to take a planar orientation, which bends the light. (The movie shows 5 seconds out of every minute for a total elapsed time of 20 minutes. Drag the slider bar to make the change more noticeable.) A black line will appear at the edge of the sensor after several minutes. The dimethyl methylphosphonate concentration has increased in the sensor and the LC is now isotropic. It will not bend light in this orientation. (The move shows 5 seconds out of every two minutes for a total elapsed time of an additional 10 minutes. Drag the slider bar to make the change more noticeable.) 4'-Pentyl-4-biphenyl-carbonitrile
Copper(II) perchlorate
Dimethyl methylphosphonate Portions of glass slides
Small petri dish with polarizer on each plate
Plastic wrap
Binder clips
Hair dryer
5µL pipets and bulb
Developed in collaboration with the
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified July 2, 2013 .
University of Wisconsin Materials Research Science and Engineering Center
Interdisciplinary Education Group | MRSEC on Nanostructured Interfaces
This page created by George Lisensky, Beloit College. Last modified July 2, 2013 .