Nanotubes and Other Forms of Carbon
Carbon is found naturally as graphite, diamond, buckyballs, and nanotubes.
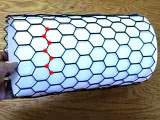
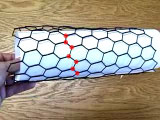
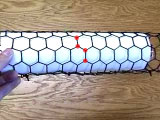
The model shows the structure of one hexagonal layer of carbon atoms, a graphene sheet. Stacks of graphene sheets form graphite, one of the allotropes of carbon. The structure easily cleaves between planes, leading to the utility of graphite writing pencils. The model shows the structure of graphite, one of the allotropes of carbon. Its structure is comprised of hexagonal layers of carbon atoms. One such hexagonal layer, shown in yellow, is called a graphene sheet. A graphene sheet can be rolled to form another allotrope of carbon called a carbon nanotube. The graphene sheet can be rolled more than one way, producing different types of carbon nanotubes.Download a pdf file with hexagonal sheets. Print on paper to roll your own nanotube. Printing this file on a plastic transparency will let you show this experiment on an overhead projector.
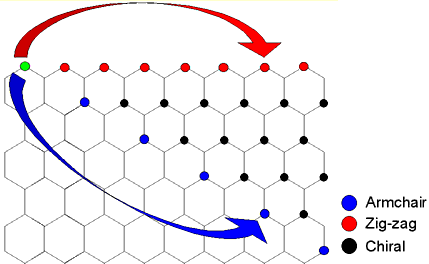
The graphene sheet can be rolled more than one way, producing different
types of carbon nanotubes. The three main types are armchair, zig-zag,
and chiral.
*Magnetic models created by Eatai Roth, Anne-Marie Nickel, and Tim Herman through the Milwaukee School of Engineering NSF-sponsored REU program using the Rapid Prototyping Center and the Center for Biomolecular Modeling.
Exploring the Nanoworld | MRSEC Nanostructured Interfaces
Copyright © 2013 The Board of Regents of the University of Wisconsin System.
This page created by George Lisensky, Beloit College. Last modified October 27, 2013 .
This page created by George Lisensky, Beloit College. Last modified October 27, 2013 .