Catalytic Converter
This type of catalytic combustor is found in the exhaust systems of automobiles and in the flue pipes of modern wood burning stoves. The active component is finely dispersed particles of platinum or palladium.
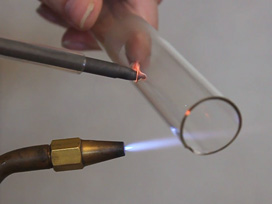
Propane gas passing through the apparatus can be lit with a match. A propane flame is used to preheat the ceramic.The flame is then extinguished but the propane continues to flow.
Propane passing over the hot catalyst exothermically oxidizes and increases the temperature of the ceramic. The gas resulting from propane passing over the hot catalyst will extinguish a lit match. How does the observed effect compare with that in the first movie where the catalyst is not hot? When the propane flow is turned off, the catalyst cools off. Why?
Materials
A glass chimney containing a piece of ceramic with a platinized surface
HS-3 Catalytic Combustor
Educational Innovations
362 Main Avenue
Norwalk, CT 06851
203-229-0730
http://www.teachersource.com
Or
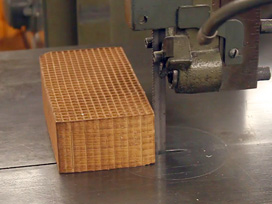
Use a band saw to cut up a Northline Express Catalytic Combustor Replacement Cut squares 3x3 or 2x2 depending on the size of the glass tubing (next step). The chimney can be made from a 25x250mm test tube. Make a scratch near the bottom. Remove the bottom by heat stress at the scratch. Fire polish the sharp edges. Indent two stops about two inches from the bottom.
A methane or propane source.
A BernzoMatic Trigger Start Torch from a hardware store is used in the
movies but a Bunsen burner also works.
Exploring the Nanoworld | MRSEC Nanostructured Interfaces
Copyright © 2013 The Board of Regents of the University of Wisconsin System.
This page created by George Lisensky, Beloit College. Last modified June 15, 2016 .
This page created by George Lisensky, Beloit College. Last modified June 15, 2016 .