Light Emitting Diodes
Unlike an incandescent bulb where a filament is heated to generate light, light is emitted in a LED when electrons, excited from their chemical bonds by electrical energy from a battery, re-form these bonds, releasing the energy as light. The process of emitting light from an LED is more rapid and more efficient than in the incandescent bulbs where most of the energy is wasted as heat. The high efficiency, fast switiching times, and low heat output of the LED have led to many new applications.
By varying their atomic compositions, light emitting diodes (LEDs) of any color can be made. Red, green, and blue light are the primary colors of additive color mixing. Varying amounts of these three colors of light can be added to create the other colors of light in the visible spectrum. Color televisions and computer monitors use this red-green-blue (RGB) additive color mixing scheme to create their images. (In contrast, paint and ink pigments rely upon subtractive color mixing of the yellow, cyan, and magenta primary colors.) We are grateful for design contributions from Tom Braniak, Trish Ferrett, and Mike Condren. An LED traffic light in Wisconsin. This one still has an incandescent yellow light.Larger movie in a new window An LED traffic light in Sweden. A Luxeon LED traffic light. These high power LEDs have a copper heat sink.
Larger movie in a new window An LED walk-don't walk traffic signal. LED lighting in the Jefferson memorial. An LED replacement for neon architectural lighting. What properties of LEDs make them suitable for this message display? An incandescent bulb (right) produces enough heat to melt butter. An LED bulb (left) does not. An incandescent bulb (right) produces enough heat to melt butter. An LED bulb (left) does not. When an LED color strip is powered by a capacitor with decreasing voltage, LEDs requiring higher energy go out first.
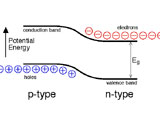
ICE Color Strip Kit When electrons and holes combine in the LED's p-n junction, light is produced.
Larger movie in a new window Use of flashing LEDs as body adornment is becoming more popular. An LED flashlight that can produce many colors.
Exploring the Nanoworld | MRSEC Nanostructured Interfaces
Copyright © 2013 The Board of Regents of the University of Wisconsin System.
This page created by George Lisensky, Beloit College. Last modified October 27, 2013 .
This page created by George Lisensky, Beloit College. Last modified October 27, 2013 .