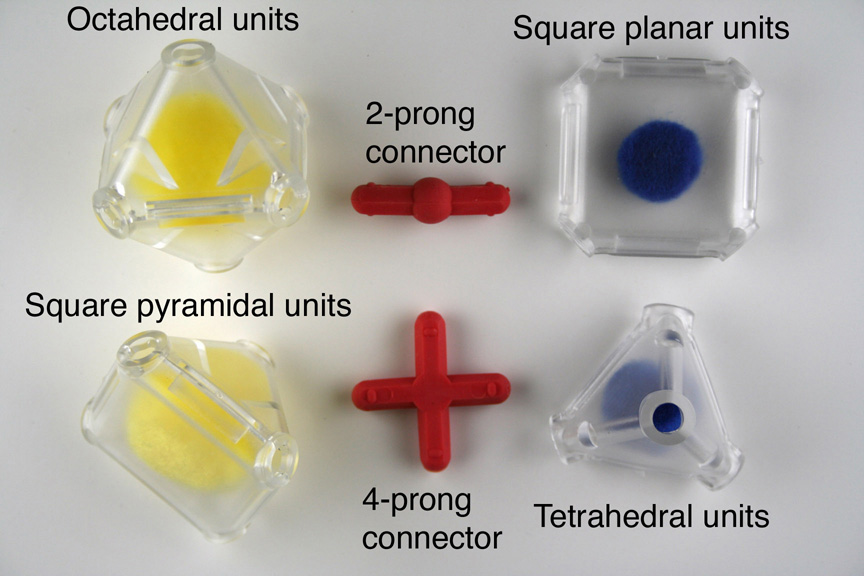
Polyhedral Model Kit
Many solids can be viewed as a close-packed
array of anions with the spaces between the anions occupied by cations.
Those spaces have tetrahedral and octahedral geometry - the same geometries
as the structural units in the polyhedral model kit.
Polyhedral models, commonly used in inorganic or solid state chemistry,
environmental or soil science, and geology, are useful for visualizing
how tetrahedral and octahedral units fit together. Because they consist
of structural units instead of individual atoms, polyhedral models can
be assembled quickly. By focusing on units larger than atoms, patterns
in complex structures can be more easily discerned.
Online interactive directions are available for over 40 structures such as NaCl, ZnS, ice, rutile,
perovksites, magnetite, gibbsite, talc, muscovite, and quartz.
The kit includes flexible connectors and pieces for assembly of 60 tetrahedral units, 78 octahedral units, 24 square pyramidal units, and 18 square planar units. Two or three kits are adequate for 20 students working in small teams, especially if larger structures are built by teams working together.
To order a kit contact the Institute
for Chemical Education.
Unfortunately the Institute for Chemical Education at the University of Wisconsin Madison shut down during COVID and has not reopened.
This kit was created by George C. Lisensky, Amy C. Payne, Kenneth L. Gentry, Ann Comins, S. Michael Condren, Wendy C. Crone and Arthur B. Ellis.