Polyhedral Model Kit
Why Polyhedra?
Most simple inorganic compounds consist of atoms that alternate indefinitely rather than existing as discrete molecules in the solid state. The formula of a molecule gives the actual number of atoms in the molecule. How do we give the number of atoms in a solid? In such cases the empirical formula is used as the chemical formula.
An example is NaCl which does not consist of just one sodium ion and just one chloride ion. Rather, in NaCl each sodium ion is surrounded by an octahedron of chloride ions and each chloride ion is surrounded by an octahedron of sodium ions in an extended structure. There are multiple ways to represent the structure of such a solid.
| The space-filling model is best for showing relative atom sizes and how the atoms fill space. | 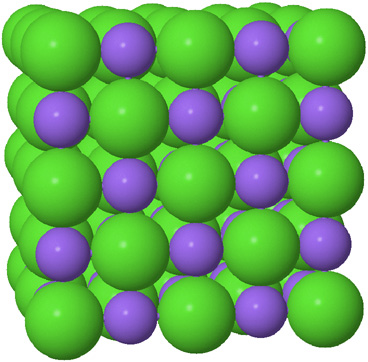 |
| The ball-and-stick model is best at showing distances and angles. The sticks do not represent localized electron bonds in this ionic solid. | 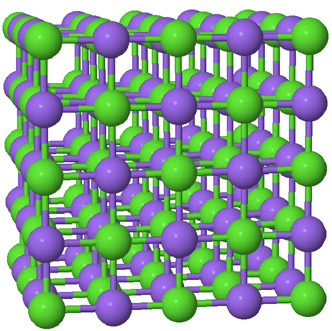 |
| The polyhedral model, where a cation surrounded by its anions is represented by a single polyhedron, is useful for visualizing how structural components fit together. Only atoms belonging to complete polyhedra are shown. | 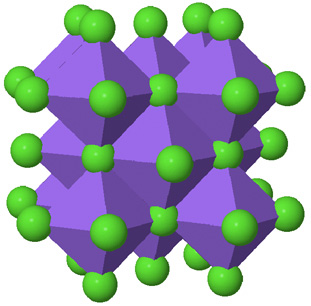 |
All of the figures on this page show the same structure at the same scale. Which is easier to describe?
This page created by George Lisensky, Beloit College. Last modified April 25, 2015 .