Ferrofluids
Introduction
In recent years, researchers have prepared ferrofluids, which have the fluid properties of a liquid and the magnetic properties of a solid. The ferrofluids actually contain tiny particles (~10 nm diameter) of a magnetic solid suspended in a liquid medium.
Ferrofluids were originally discovered in the 1960s at the NASA Research Center, where scientists were investigating different possible methods of controlling liquids in space. The benefits of a magnetic fluid were immediately obvious: The location of the fluid could be precisely controlled through the application of a magnetic field, and, by varying the strength of the field, the fluids could be forced to flow. Researchers have prepared ferrofluids containing small particles of ferromagnetic metals, such as cobalt and iron, as well as magnetic compounds, such as manganese zinc ferrite, ZnxMn1-xFe2O4. (0 < x < 1; this is a family of solid solutions). But by far, the most work has been conducted on ferrofluids containing small particles of magnetite, Fe3O4.
How they work
Ferrofluids containing magnetite can be prepared by combining the appropriate amounts of an Fe(II) salt and an Fe(III) salt in basic solution, a combination that causes the mixed valence oxide, Fe3O4, to precipitate from solution:
2 FeCl3 + FeCl2 + 8 NH3 + 4H2O --> Fe3O4 + 8 NH4Cl
However, the particles of magnetite must remain small in order to remain suspended in the liquid medium. To keep them small, magnetic and van der Waals interactions must be overcome to prevent the particles from agglomerating. Thermal motion of magnetite particles smaller than ~10 nm in diameter is sufficient to prevent agglomeration due to magnetic interactions.
The van der Waals attraction between two particles is strongest when the particles approach each other at close distances. Therefore, one method of preventing agglomeration due to van der Waals and magnetic forces is to keep the particles well separated. This separation can be accomplished by adding a surfactant to the liquid medium. The surfactants can generate either steric or electrostatic repulsions between the magnetic particles. For example, cis- oleic acid can be used for oil-based ferrofluids as a surfactant that produces steric repulsions. The surfactant is a long-chain hydrocarbon with a polar head that is attracted to the surface of the magnetite particle; thus a surfactant coating is formed on the surface. The long chains of the tails act as a repellent cushion and prevent the close approach of other magnetite particles (Figure 1).
A. 2 FeCl3 + FeCl2 + 8 NH3 + 4H2O --> Fe3O4 + 8 NH4Cl
B. Add cis-oleic acid, CH3(CH2)7CH=CH(CH2)7COOH, in oil.
C. Remove water:
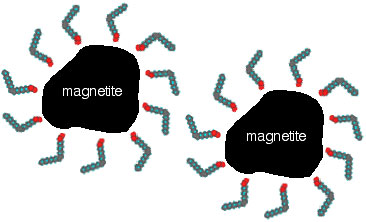

Figure 1. A preparation of ferrofluid: (A) synthetic conditions for production of Fe3O4(s); (B) addition of surfactant; and (C) removal of water to give small particles of Fe3O4 stabilized by the interaction of the polar ends of the oleic acid molecules with the magnetite particles, and by the interaction of the nonpolar ends of the oleic acid molecules with the oil serving as the liquid medium.
Ionic surfactants such as tetramethylammonium hydroxide can be used as a surfactant that produces electrostatic repulsion in an aqueous medium. The hydroxide ions are attracted to the surface of each magnetite particle, forming a negatively charged layer at the magnetite surface. The tetramethyl ammonium cations are attracted to the negatively charged layer, forming a positive layer. When magnetite particles approach each other the repulsions between their positively-charged layers keeps them from getting too close. (Figure 2).
A. 2 FeCl3 + FeCl2 + 8 NH3 + 4H2O --> Fe3O4 + 8 NH4Cl
B. Replace excess NH4OH on Fe3O4 surface with N(CH3)4OH
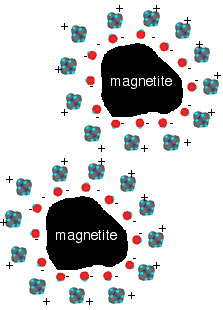
Figure 2. A preparation of ferrofluids: (A) the synthetic conditions for production of Fe3O4(s); and (B) addition of surfactant to give small particle of Fe3O4 (shaded circles) stabilized by interaction of the hydroxide anions with the magnetite and by the interactions of the tetramethylammonium cations with the water serving as the medium.
Some ferrofluids are so attracted to magnetic fields that they will stand up along magnetic field lines, forming an array of spikes (Figure 3).


Figure 3. Top and side views of the magnetic spiking phenomenon observed when a cow magnet is placed beneath a Petri dish containing a ferrofluid. The ferrofluid aligns with the magnetic field lines of the magnet to produce the spikes.
Applications
Although the array of spikes on the surface of the ferrofluid is spectacular, this property is not particularly useful. However, ferrofluids have found a wide variety of applications, including use in rotating shaft seals. A ferrofluid can behave as a liquid O-ring where a rotating shaft enters either a low- or high-pressure chamber. The ferrofluid is held in place by permanent magnets and forms a tight seal, eliminating most of the friction produced in a traditional mechanical seal. These rotating shaft seals are found in rotating anode X-ray generators and in vacuum chambers used in the semiconductor industry. Ferrofluid seals are used in high-speed computer disk drives to eliminate harmful dust particles or other impurities that can cause the data-reading heads to crash into the disks.
Another application of ferrofluids is in improving the performance of loudspeakers. In a loudspeaker, electric energy is sent through a coil located in the center of a circular permanent magnet. The magnetic field induced by the electric energy causes the coil to vibrate and thus produces sound and heat. Bathing the electric coil in a ferrofluid, which is held in place by circular permanent magnets, dampens unwanted resonances and also provides a mechanism to dissipate heat from excess energy supplied to the coil. Both of these factors lead to an overall improved sound quality.
Finally, there is much hope for future biomedical applications of ferrofluids. For example, researchers are attempting to design ferrofluids that can carry medications to specific locations in the body through the use of applied magnetic fields. Other ongoing work is investigating the use of ferrofluids as contrast agents for magnetic resonance imaging (MRI).
References
Adapted from Teaching General Chemistry: A Materials Science Companion by A. B. Ellis, M. J. Geselbracht, B. J. Johnson, G. C. Lisensky, and W. R. Robinson. Copyright © 1993, American Chemical Society, Washington, DC.





