Amorphous Metals
Introduction
What is a crystalline material?
Most metals, including stainless steel, have a crystalline structure. This means that the atoms in the structure arrange themselves in an ordered manner, in which a small repeat unit called a “unit cell” can be identified. This unit cell, which in some cases contains just several atoms, is repeated in all three directions, and in this way, the entire structure is built up.
This unit cell description of a crystalline structure implies the atoms are arranged in perfect order, which is only true in an ideal solid. All crystalline solid structures contain missing atoms, called defects, impurity atoms of other elements, and misaligned planes of atoms called dislocations.
Dislocations are rather common in many systems you encounter everyday. For example, look at the corn cob pictured below. Can you see how one row of kernels has been inserted into the regular arrangement of rows? This is called a dislocation. This same type of thing occurs in the arrangement of atoms in a crystalline solid.

Impurity atoms, defects, and dislocations all have an important impact on the physical and chemical properties of the solid. For example, copper wire is easy to bend because the structure contains planes of atoms which can slip easily past one another. Watch the movie below to see how a model of the structure of copper metal contains these planes of atoms which slip easily apart.
How they work
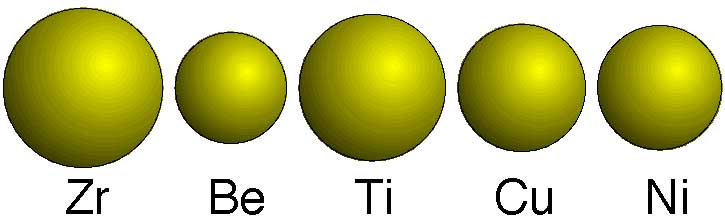
The atoms in an amorphous material are not arranged in any ordered structure, rather they have a tightly-packed, but random arrangement. Amorphous materials are formed by cooling the liquid material quickly enough to prevent crystallization; the atoms do not have time to arrange themselves into an ordered structure. Liquidmetal® is an amorphous alloy (also known as a metallic glass) containing five elements, with the elemental composition is 41.2% zirconium, 22.5% beryllium, 13.8% titanium, 12.5% copper, and 10.0% nickel.
Because of the varying sizes of these atoms, and their random arrangement in the solid, there are no groups of atoms that can easily move past one another. Because there are no planes of atoms in an amorphous material, the atoms are gridlocked into the glassy structure, making the movement of groups of atoms very difficult. One consequence of this atomic gridlock, is that some amorphous metals are very hard. Liquidmetal® is more than two times harder than stainless steel. However, besides being a very hard material, this amorphous alloy has a low elastic (or Young's) modulus. The combination of hardness and elasticity of Liquidmetal® is an important factor in its many applications.
Applications
Golf clubs with amorphous metal heads, created by Liquidmetal®, have been on the market since 1998.
Industrial coatings for equipment and machinery that are exposed to environments of high wear, temperature, and corrosion. Amorphous metal has the lowest coefficient of friction of any metallic coating, and significantly extends part lifetime. Example: the wall of a refinery coker.
Armor-piercing ammunition that enhances the performance and safety levels for users. The military is developing armor-piercing ammunition that uses amorphous metal instead of Depleted Uranium (DU) alloy.
Casings for electronics and telecommunications equipment, such as cellular handsets, that are stronger, smaller, and thinner.
Amorphous metal knives are used in medical fields, such as ophthalmic medicine, because they are sharper than steel, less expensive than diamond, and higher quality than diamond; they are more consistently manufactured than steel or diamond; and they have longer lasting blades.
Solar wind collector tiles on NASA's Genesis spacecraft, the first mission to collect and return samples of the solar wind. The tiles are comparable to a coffee cup lid, and will play a key role in the collection process. The mission is designed to measure the composition of isotopes in solar matter. For information see: http://www.lpi.usra.edu/meetings/lpsc2000/pdf/1783.pdf
References
K. J. Nordell, N. D. Stanton, G. C. Lisensky & A. B. Ellis. The "Atomic Trampoline" Kit: Demonstrations with Amorphous Metal (ICE, Madison, WI, 2000).
Useful links:
- http://www.liquidmetaltechnologies.com has further information about general applications.



