

Nuclear Magnetic Resonance
Carbon-13 NMR
The first carbon-13 studies were conducted in 1957. However, the widespread use of this technique did not occur until the 1970s, because of the weak signal that resulted from the low natural abundance, 1.11%, of this isotope and its low magnetogyric ratio. These factors make C-13 NMR about 6000 times less sensitive than proton NMR.
Stronger magnetic fields have helped to overcome the problem of weak C-13 signals. Multiple scan spectra and the use of Fourier transformations of the time domain spectra have also contributed to the enhancement of the C-13 signals.
Carbon-13 NMR is a powerful complement to proton NMR, because it examines the carbon backbone of molecules. There is no splitting of peaks due to the presence of neighboring carbon atoms, because the low probability of two C-13 atoms adjacent to one another is very low, and C-12 atoms have a spin quantum number of zero. The chemical shifts for C-13 are typically more than 10 times greater than for H-1, resulting in less overlap of peaks. The heteronuclear spin coupling of C-13 and H-1 atoms can be eliminated using any one of several spin decoupling techniques. The resulting spectrum can exhibit one peak for each type of carbon atom.
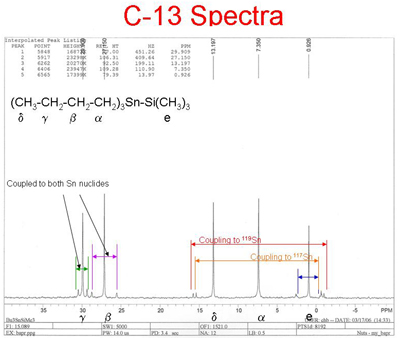
Click here for larger image
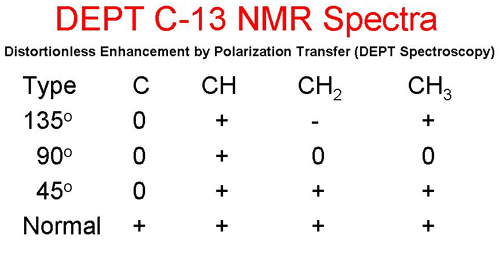
Distortionless Enhancement by Polarization Transfer (DEPT Spectroscopy) is a powerful tool for determining the classification of the carbon atoms: primary (CH3), secondary (CH(2), tertiary (CH), or quarternary (C). In the following table, a + sign indicates that the peak will be pointing upward, a - indicates that the peak will be pointing downward, and a 0 indicates that the peak disappears.
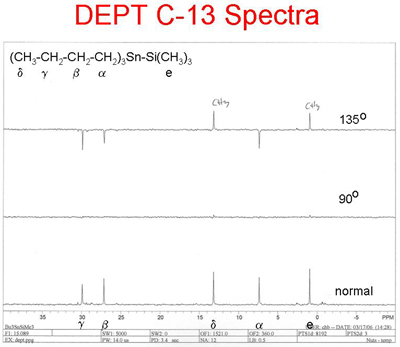
The following spectrum represents a DEPT spectrum of the compound shown above. The lowest spectrum is a normal decoupled broad band C-13 spectrum. The middle spectrum represents the so called 90o spectrum, and the top spectrum represents the so called 135o spectrum.
Click here for larger image
The normal spectrum shows 5 C-13 peaks indicating that their are 5 distinct types of carbon atoms in the structure. The 90o spectrum shows no peaks indicating that there are no tertiary carbons. The 135o spectrum shows a total of 5 peaks 2 pointing upward, primary (methyl) carbons, and 3 pointing downward secdonary (methylene) carbons.
<NMR Instruments] | [Other NMR Nuclei>