Education and Outreach: Nanotechnology Activity Guides
"Smart" Papers
Audience: Middle school class
Time Needed: 50 minutes
Objectives:
- Introduce microencapsulation technology, used in "smart" paper
- Understand different methods to break microcapsules: heat, pressure and dissolution
- Learn the mechanics of how "smart" paper uses invisible ink
- Realize that "smart" paper is widely used in every day life, employing common examples such as tickets and receipts.
Related Wisconsin Model Academic Science Standards:
- A.8.6 Use models and explanations to predict actions and events in the natural world
- C 8.1 Identify questions they can investigate using resources and equipment they have available
- D.8.3 Understand how chemical interactions and behaviors lead to new substances with different properties
- D.8.4 While conducting investigations, use the science themes to develop explanations of physical and chemical interactions and energy exchanges
- ELA C.8.3 Participate effectively in discussion
- G.8.3 Illustrate the impact that science and technology have had, both good and bad, on careers, systems, society, environment and quality of life
Activity Materials:
- Carbonless copy paper (enough for each student to have a ~2" x 2" square)
- Pen/pencil
- Lemon juice and small container to hold lemon juice
- Absorbent paper (hand made paper is best, water color paper also works well) (enough for each student to have an ~2" x 2" square
- Cotton swabs (one per student)
- Newspaper or trays (to protect work space)
- Thermal paper (enough for each student to have an ~2" x 2" square)
- Heat source (iron or toaster)
- Bath beads (several, if splatter box is being utilized)
- Paintballs (several, if splatter box is being utilized)
- Paintball "splatter box" and weights (or Bubble wrap can be used to illustrate the same concepts)
- Red cabbage juice
- Spray bottle
- Computer paper
- Examples of thermal paper (receipts, airline tickets, concert tickets, lottery tickets)
Activity Instructions:
Starting Points
Carbonless copy paper functions when the coated backs and fronts match up. This allows the invisible ink to transfer from the top sheet to the bottom sheet and develop color. By exploring possible orientations of a three-sheet copy system, the students will realize this requirement. A generic clothes iron produces a dramatic color change upon contact with a sheet of thermal paper. In order to prove this is not the paper burning, the same procedure is performed on a sheet of generic computer paper.
 Different
cylindrical weights are dropped onto a paintball or a bath bead placed inside
a 1 ft3 Plexiglas box (see picture at right). Depending on the weight
used, the capsule will burst or will remain intact. This demonstrates the different
forces carbonless paper comes in contact with during its lifetime. The heavier
weights represent the use of a writing utensil, and the lighter weights represent
pressure experienced during packing and shipping.
Different
cylindrical weights are dropped onto a paintball or a bath bead placed inside
a 1 ft3 Plexiglas box (see picture at right). Depending on the weight
used, the capsule will burst or will remain intact. This demonstrates the different
forces carbonless paper comes in contact with during its lifetime. The heavier
weights represent the use of a writing utensil, and the lighter weights represent
pressure experienced during packing and shipping.
Once the invisible ink inside the capsule is released by pressure or heat, it combines with other components in the opposing sheet (if carbonless paper) or within the sheet (if thermal paper) and reacts to form an image. To help convey this idea, the students perform an acid-base indicator reaction (lemon juice-red cabbage juice). As an analogy to the thermal paper reaction, a clothes iron is applied to a sheet of paper which has an image drawn in lemon juice, which forms color as well.
A final challenge is issued to the students to combine what they have learned in the activity. They are asked to come up with the necessary tools and possible methods of differentiating between smart paper (either carbonless or thermal) and regular paper.
Activity
Give each student two sheets of absorbent paper and ask them to label one side with regular ink. On the same side, have them use the cotton swab to apply the lemon juice to both sheets in any design, message, or pattern. Allow the paper to dry for a minimum of 15 minutes. Once the papers are dry, have the students apply heat to one of the sheets by using the iron or toaster and observe the colored image. Then have the students to apply red cabbage juice (using a spray bottle) to the other sheet, again observing a formed image.
Give the students 3 different sheets of carbonless paper and ask them to determine the order necessary to result in transfer of the image between first sheet and other two. Remind them that the order of the sheets is important.
Take one sheet of plain white paper and one sheet of plain white thermal paper and hold the iron on each sheet of paper for the same amount of time. Notice the appearance of a dark shape on the thermal paper. Explain to the students that the lack of an image on the plain white paper demonstrates that the color change is not the paper burning.
Position a paintball in the center of splatter box. Have students drop various cylinders of different weights and compositions; note which cylinders rupture the paintball and which do not. Repeat this procedure with a bath bead in place of the paintball. Or if splatter box is not available give students a small piece of bubble wrap, ask them to pop a bubble and ask how they popped it. Have them imagine what would have happened if ink were present in the bubble.
Divide students into groups and give each group a random sample of various papers, some regular, some smart. Each group should discern the type of technology (carbonless, thermal, or none) that is present.
The activity can finish by students working together on the Smart Paper Worksheet and discussing the answers as a class.
Required Background Information:
Carbonless copy paper consists of 3 different types of paper, coated back (CB) coated front and back (CFB) and coated front (CF). You can also find self contained copy paper (SC). Carbonless copy paper is coated with a variety of chemicals, CB sheets have a layer of microcapsules, microscopic capsules, that contain an invisible ink. The CF sheet has a coating of a coreactant, which when exposed to the colorless inks in the microcapsules reacts with them to produce color.
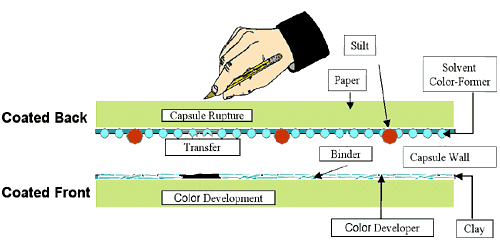
The benefit of the microcapsules is that they keep the reactants away from each other until you want color to develop, which is when you write on the top sheet of paper. Writing on the top sheet produces mechanical pressure that bursts the microcapsules, allowing them to release their colorless ink leading to development of color on the facing sheet of paper. Alternate applications of microcapsules can include delivery of drugs and scratch and sniff stickers.
Students should have some understanding of chemical reactions before the presentation. The important concept is that two things coming in contact with each other can sometimes change each other. Most students have probably seen a vinegar and baking soda volcano, this is one form of a chemical reaction as is the glow stick.
The red cabbage and lemon juice is an acid base reaction. The red cabbage juice acts as a pH indicator and changes color when exposed to acid (pink) or base (blue), (lemon juice is acidic). The reaction that occurs when you hold an iron to the lemon juice writing is burning. Since the lemon juice burns at a lower temperature than the paper you are able to see the burnt lemon juice against the paper.
Thermal paper was developed in the late 1950s as a response to NASA's need for a printer the size of a cigar box. When it was first developed, thermal paper had a negative reputation for its fragility. Now thermal paper has been developed for a much broader range of applications and can be specifically designed to withstand harsh environments. Technically, thermal paper is a heat sensitive medium through with images or designs are produced by the application of heat energy.
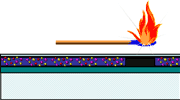 Thermal
paper is comprised of three layers: a base sheet, a base coating, and a thermosensitive
coating. The thermosensitive coating contains a colorless dye (the color former),
a bisphenol or acidic material (the color developer), and a sensitizer. Dyes
are typically basic substances which become colored when oxidized by acidic
compounds or bisphenol compounds. In these dye-developer systems, sensitizers
are typically mixed with the dyes to form a blend with a reduced melting point.
This reduces the amount of heat necessary to melt the dye and obtain reaction
with the color developer. The components of the thermosensitive coating are
often determined by the operating requirements. These components are sensitive
to the environment (heat, humidity and pressure) and many materials (organic
solvents, cleaners, petroleum solvents, ammonia, some oils, plasticizers). The
main type of thermal printing is known as Direct Thermal printing, where heat
is generated in the printhead which activates the ink in the paper to develop
color.
Thermal
paper is comprised of three layers: a base sheet, a base coating, and a thermosensitive
coating. The thermosensitive coating contains a colorless dye (the color former),
a bisphenol or acidic material (the color developer), and a sensitizer. Dyes
are typically basic substances which become colored when oxidized by acidic
compounds or bisphenol compounds. In these dye-developer systems, sensitizers
are typically mixed with the dyes to form a blend with a reduced melting point.
This reduces the amount of heat necessary to melt the dye and obtain reaction
with the color developer. The components of the thermosensitive coating are
often determined by the operating requirements. These components are sensitive
to the environment (heat, humidity and pressure) and many materials (organic
solvents, cleaners, petroleum solvents, ammonia, some oils, plasticizers). The
main type of thermal printing is known as Direct Thermal printing, where heat
is generated in the printhead which activates the ink in the paper to develop
color.
Supplemental Materials:
- Teacher's Guide: Materials and where to find them (pdf)
- Teacher's Guide: Extended Activity Instructions (pdf)
- Handout: Worksheet (pdf)
-
Intructions for making red cabbage juice(from the Miami Museum of Science website - http://www.miamisci.org/ph/phcabbage.html)
Chop a red cabbage into many small pieces. Boil the cabbage in enough water to cover the pieces, until the water becomes almost bluish, or a very deep purple. Cool the water before putting it into the spray bottle. Cabbage juice must be kept refrigerated when not in use.
References:
- Appleton Paper - http://www.appletonideas.com
- Austin, R.A., B.A. Neeld, P.C. Yao, J.K. Rourke, T.J. Amick, and W.W. McCarty. Dual layer self-contained paper incorporating hollow spherical plastic pigment. USA Patent 6,514,909. Date Issued: Feb. 4, 2003.
- Blythe, D., D. Churchill, K. Glanz, and J. Stutz. 1999. Microcapsules for Carbonless Copy Paper, p. 421-439, In R. Arshady, ed. Microcapsules and Liposomes, Vol. 1: preparation and chemical applications. Citrus Books
- Green, B.K. Pressure sensitive record material. USA Patent 2,712,507. Date Issued: July 5, 1955.
- Halbrook, Jr., Wendell B., Wehr, Mary Ann. Thermal paper with security features. USA Patent 6,562,755, Date Issued: May 13, 2003.
- Minowa, Masahiro, Imai, Satoru. Printing system, thermal printer, printer control method, and data storage medium. USA Patent 6,606,107, Date Issued: August 12, 2003
- Sadao Ishige, K., H. T., and K. H. Recording sheet employing an aromatic carboxylic acid. USA Patent 3,843,383. Date Issued: Oct. 22, 1974.
Authors:
IPSE Interns: Lynn Diener, Brian McCall
IPSE Leadership Team: Wendy C. Crone, J. Aura Gimm, Wendy deProphetis, Greta Zenner, and Tom Derenne
| Return to this activity's summary page |
The Nanotechnology Activity Guides are a product of the Materials Research Science and Engineering Center and the Internships in Public Science Education Project of the University of Wisconsin - Madison. Funding provided by the National Science Foundation.