Education and Outreach: Nanotechnology Activity Guides
Nanoarchitecture: Forms of Carbon
Audience: Middle school class
Time Needed: Two days
Day 1: 30 minutes (pre-activities)
Day 2: 45-90 minutes; modular format allows you to adjust time
Objectives:
- Be introduced to the four forms of carbon, including two new forms of carbon, fullerenes and nanotubes
- Be able to relate atomic and molecular structure to the properties of a material
Related Wisconsin Model Academic Science Standards:
- A.8.6 Use models and explanations to predict actions and events in the natural world
- C.8.5 Use accepted scientific knowledge, models, and theories to explain their results and to raise further questions about their investigations
- D.8.2 Use the major ideas of atomic theory and molecular theory to describe physical and chemical interactions among substances, including solids, liquids, and gases
- G.8.2 Explain how current scientific and technological discoveries have an influence on the work people do and how some of these discoveries also lead to new careers
Activity Materials:
- Day 1
- Pre-Activity supplemental power point transparencies
- "Yes" and "No" signs and a set of letters that spells
- "carbon" for each team of 3-4 students
- A small prize for each team
- Day 2
- Pictures or plastic models of the atomic structure of the four forms of carbon
- Mirrors, paper, pencils, and diamond scribe tips
- Team worksheets and their accompanying materials:
Friction Worksheet: balance, balls, shoe box, weight
Fullerene Worksheet: model pieces, posterboard
Nanotube Worksheet: nanotube papers, nanotube pencils
Strong Man Worksheet: paper, brick
- Posterboard or large sheets of paper and markers
Activity Instructions:
Break the students into teams of three to four and have them sit at separate tables. Many of the activities require the students to work in these teams.
Day 1 - Is This a Form of Carbon Game (15 minutes):
This activity can be used either as an introduction to find out what students
know about carbon or as a review after the team activities that investigate
the four forms of carbon. Each team has a set of letters that spells the word
"carbon"; the letters should be laid out on the floor as shown below:
| C | A | R | B | O | N |
Each letter represents a correct answer to a question in the list below. Each
team will designate a team leader, who starts by standing on the letter C. The
educator will ask the question, "is this a form of carbon?" and the
team leader will advance one letter each time the team answers
correctly. Each team has a set of "YES" and "NO" signs to
facilitate answers to the educator's questions. As each team reaches the letter
"N" the team claims a prize. The goal is to have all teams finish.
Hold up pictures of the following materials and ask the class if the materials are forms of carbon.
- A diamond ring [YES]
- Pencil lead - it's really graphite [YES]
- Table sugar [NO] The other elements found in sugar besides carbon are hydrogen and oxygen.
- Water [NO] Water consists of the elements hydrogen and oxygen.
- Coal [NO] Coal also contains hydrocarbons made from hydrogen and carbon.
Other statements used to review information:
- Are fullerenes forms of carbon? [YES]
- Are nanotubes a form of carbon? [YES]
- Is carbon the 6th element on the periodic table? [YES]
- Is the elemental symbol for Carbon C? [YES]
- Can graphite be turned into diamond through the application of heat and pressure? [YES]
- Is graphite a stronger material than diamond? [NO] Diamond is the hardest natural material known.
- Is graphite used as pencil lead? [YES]
- Could diamond be used to write on paper? [NO] The atomic structure of diamond is too rigid.
For a fun game on the internet called "Carbon is 4 Ever," visit http://library.thinkquest.org/C005377/
Day 2 - Introduction to the Four Forms of Carbon (10 minutes):
Introduce the students to the four molecular models of graphite, diamond, fullerenes,
and nanotubes. Point out that each black plastic piece stands for one carbon
atom, and the clear plastic tubes represent bonds between the atoms. Have the
students compare and contrast the shapes of the models and the arrangement of
atoms. The following ideas and questions could be addressed: Which model looks
like a soccer ball (or like Mitchell Park Climate Domes)?
How many sides does a hexagon have?
Which model(s) contain hexagons?
How many sides does a pentagon have?
Which model(s) contain pentagons?
Which model is shaped like a tube?
Summarize the structures in the following way:
Graphite: layers of sheets
Diamond: box-like shape
Fullerene: ball-shaped
Nanotube: tubular
Ask the students if they think the atomic structure of a material affects its
properties. Briefly
discuss answers.
Have the mirrors, the diamond scribe tips, and the pencils ready. Ask a volunteer
to try to write on the mirror with a pencil. Have another volunteer try to write
on the mirror with the diamond scribe tip. Ask the students to describe and
to explain their observations. The
diamond scratches the mirror because of its hardness, which is a result of its
“strong” atomic structure. Ask the students to look at the diamond
model and see if they can figure out what makes this form of carbon so strong.
If they need help, talk about the following: each atom is
tightly bonded to four other carbon atoms. This makes a tri-pod-like shape called
a tetrahedron, which is very strong. Ask the students why they think graphite
writes on paper.
Again, have them use the model for ideas. If they need help, explain that graphite can easily “slide off” onto the paper because of the layered structure formed by sheets of carbon atoms. Ask the students again if they think the atomic structure of a material affects its properties.
Team Work (25 minutes):
Hand out a worksheet packet to each team (Worksheets 1-6) along with the corresponding
materials. Each team will only work on one worksheet packet, so each team will
be learning about different properties of carbon. Hand out “Hints”
to the teams as the students ask for
them.
Summaries of team worksheets:
Worksheet 1. Name that Nanotube
Nanotubes have three different structures: chiral, zig-zag, and armchair. Students learn
to distinguish between the different types of nanotubes by studying nanotube
structures printed on pencils (which have the same tubular shape as nanotubes).
Worksheet 3: Fun with Fullerenes
Fullerenes are soccer-ball-shaped molecules of carbon atoms. Students create
a model of a fullerene that consists of 12 pentagons and 20 hexagons.
Worksheet 5. Strong Man Contest
Nanotubes are five times stronger than steel. Students analyze how the tubular shape
of nanotubes makes them very strong.
Worksheet 6. Fun with Friction
Fullerenes are good lubricants due to their spherical shape. Students compare
the lubricating properties of the forms of carbon through a series of experiments.
Preparing Posters (15 minutes):
Give each team a piece of posterboard and allow the students time to create
a poster based upon what they have learned by going through their worksheet
packet.
Presenting Posters (10 minutes):
Allow each team 2-3 minutes to share its poster with the rest of the class.
Ask the class the following question: “Does the structure of a material
effect its properties?”
Required Background Information:
Atoms are the building blocks of everything, and the elements are different
kinds of atoms. Of all the elements that exist, carbon is found in all living
things and is one of the most abundant elements on our planet. Carbon atoms
can arrange themselves in several different ways to create
different materials with varying properties.
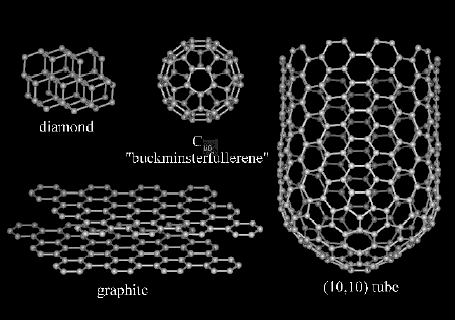
In diamond, the carbon atoms are connected to each other in all three dimensions,
making it a very hard material. Graphite consists of layers of graphene sheets,
layers of hexagonally patterned carbon atoms, which form a two-dimensional structure.
The layers easily slip off a
pencil tip when we write. The two-dimensionality of graphite makes it a softer
material. Most middle school students have started to learn about atoms and
may even know about two forms of carbon (graphite and diamond), but they probably
are not aware of two new forms of
carbon, fullerenes and nanotubes. Fullerenes consist of a caged structure similar
to the shape of a soccer ball (or the Epcot dome at Disney World). Nanotubes
are a fourth form of carbon that is made up of rolled graphene sheets to create
a tubular shape.
Supplemental Materials:
- Handout: Student Worksheets [ Friction | Fullerene | Nanotube | Strong Man ](pdf)
- Handouts: Nanotube Papers [ Armchair | Chiral | Zig-zag ] (pdfs)
- Handouts: Other Activities [ Word Search 1 | Word Search 2 | Crossword Puzzle ] (pdfs)
- Carbon Nanotube Activity Guide (pdf)
References:
- “Carbon Nanotube Actuators” Baughman, R. et al. Science 1999, 284, 1340-1344.
- Carbon Nanotube Movie - http://mrsec.wisc.edu/edetc/cineplex/nanotube/index.html
- Carbon Nanotubes - http://www.ipt.arc.nasa.gov/carbonnano.html
- Columbia University, "Our Energy Challenge" video - http://smalley.rice.edu
- IBM Research Nanoscale Science Department: Carbon Nanotubes - http://www.research.ibm.com/nanoscience/nanotubes.html
- “Starched Carbon Nanotubes” Star, A.; Steuerman, D.; Heath, J.; Stoddart, F. Angewandte Chemie, International Edition 2002, 41, 2508-2512.
- The Nanotube Site - http://nanotube.msu.edu
Authors:
IPSE Interns: Wendy deProphetis, Ed Kabara,
Naveen Varma
IPSE Leadership Team: Wendy C. Crone, Amy Payne, Greta Zenner, and Tom Derenne
| Return to this activity's summary page |
The Nanotechnology Activity Guides are a product of the Materials Research Science and Engineering Center and the Internships in Public Science Education Project of the University of Wisconsin - Madison. Funding provided by the National Science Foundation.