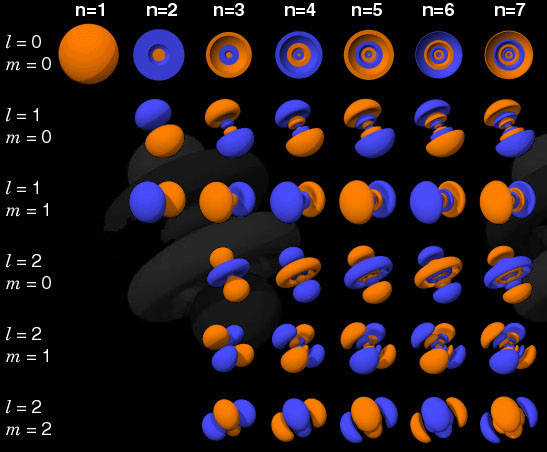
The electron orbitals presented here represent a volume of space within which
an electron would have a certain probability. For example, in a simple lowest-energy
state hydrogen atom, the electrons are most likely to be found within a sphere
around the nucleus of an atom. In a higher energy state, the shapes become
lobes and rings. With the exception of the n = 1 orbital, all orbitals
in the top row are cutaway to show the concentric spheres. For more details
and a larger collection, see http://www.albany.net/~cprimus/self/orbtable.htm
Some hydrogen orbitals | What's in a Star? | ChemConnections